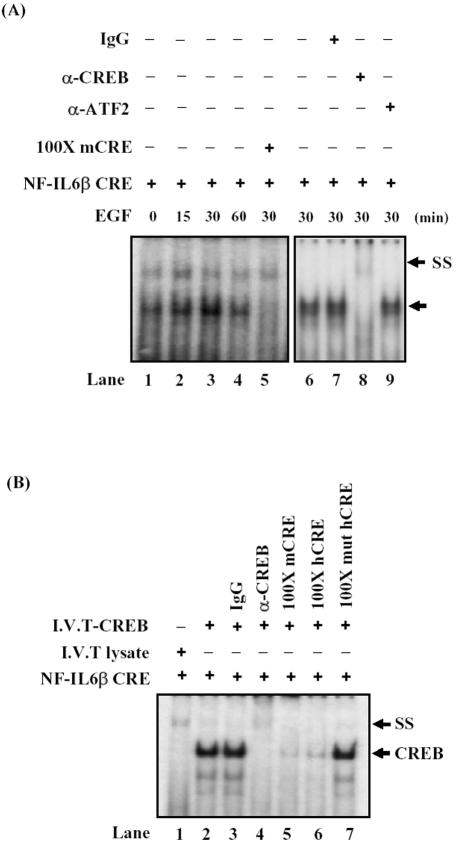
Figure 3.
CREB was one component of the CRE-binding complex and directly bound to NF-IL6β promoter in vitro. (A) In lanes 1–4, nuclear extracts from A431 cells deprived of serum and restimulated with EGF for various time courses were incubated with the 32P-labeled CRE element of NF-IL6β gene. In lane 5, competition analysis was carried out by the addition of an 100-fold molar excess of unlabeled mCRE oligonucleotides. In lane 6, no antibody was present in the binding reaction. In lanes 7, 8 and 9, binding assays were performed with control, CREB, or ATF2 specific antibody, respectively. The solid arrow indicates the specific DNA-protein complex, and the “SS” arrow indicates the band supershifted by the antibody. (B) The CREB protein synthesized in vitro by the TNT-coupled reticulocyte lysate system was allowed to bind to the CRE probe (lane 2). Incubation with a control rabbit IgG (lane 3), purified rabbit anti-CREB antibodies (lane 4), excess unlabeled mCRE (lane 5), hCRE (wt, lane 6) or hCRE-mutant (muthCRE, lane 7), were performed respectively, and then resolved on the nondenaturing gel. “CREB” represents the CREB retard shifting, and “SS” indicates the supershifted band by antibodies.