Abstract
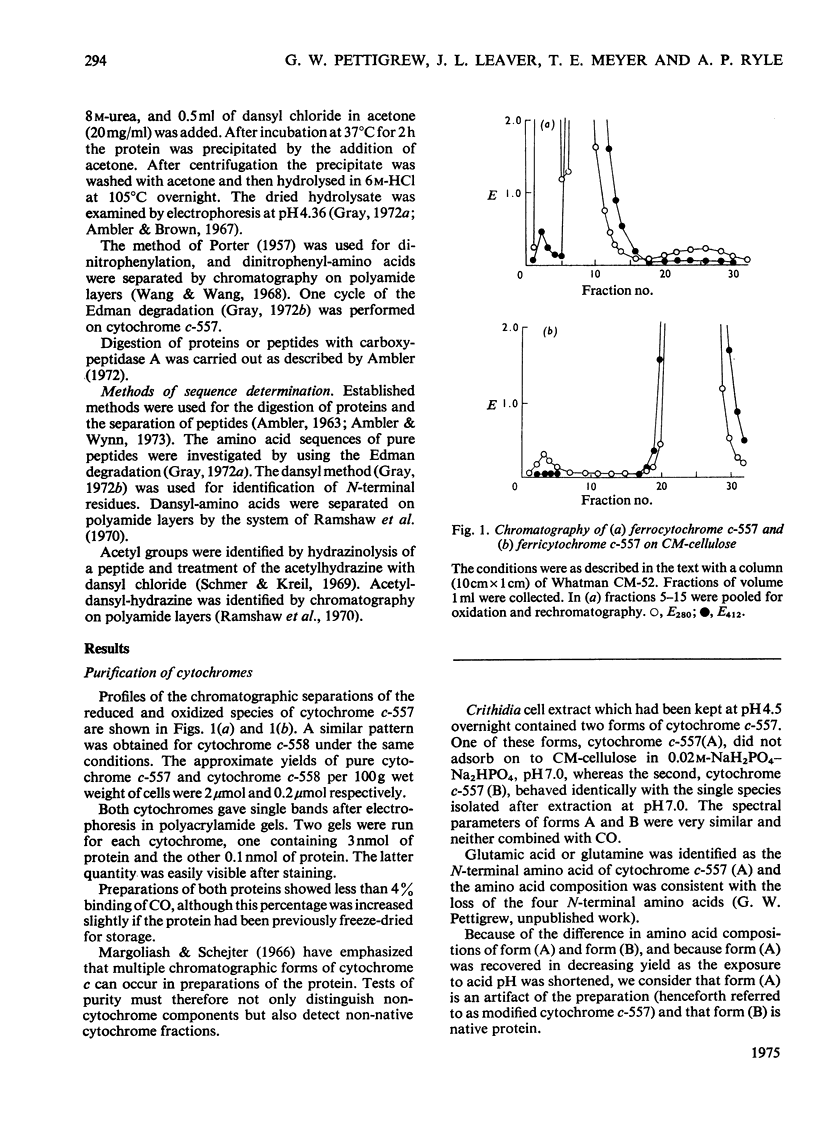
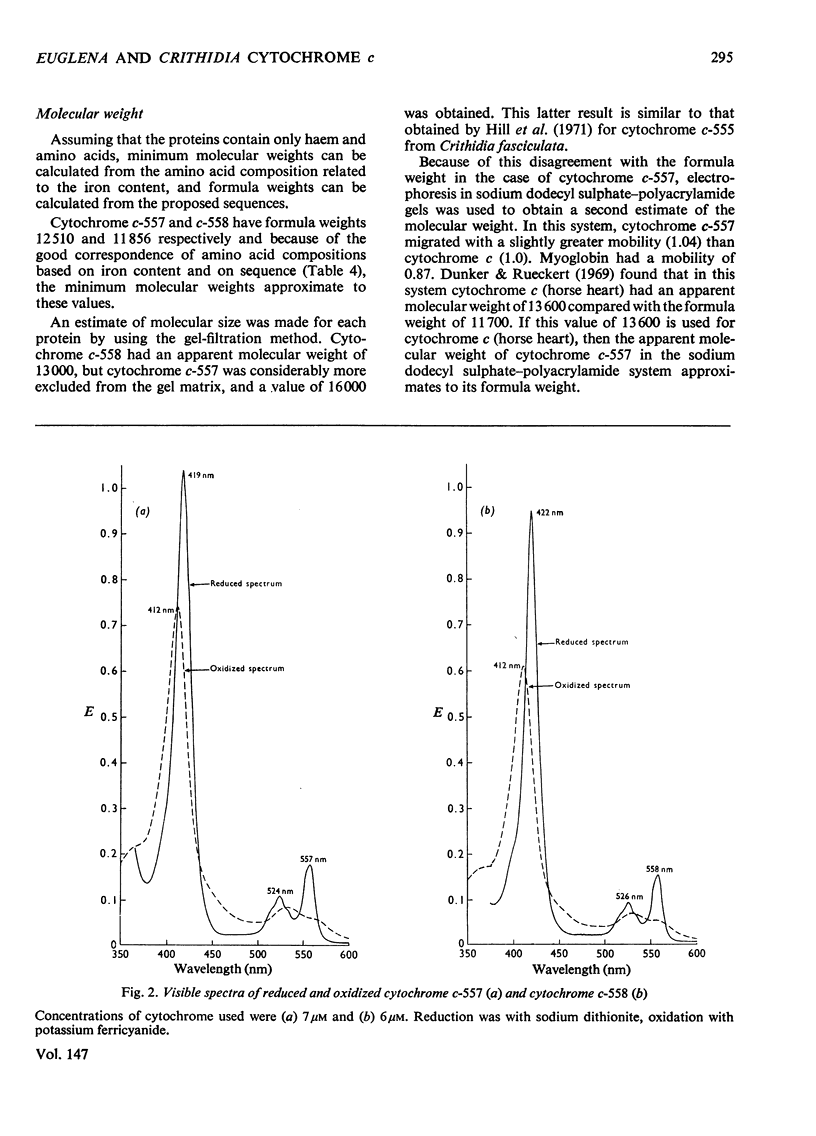
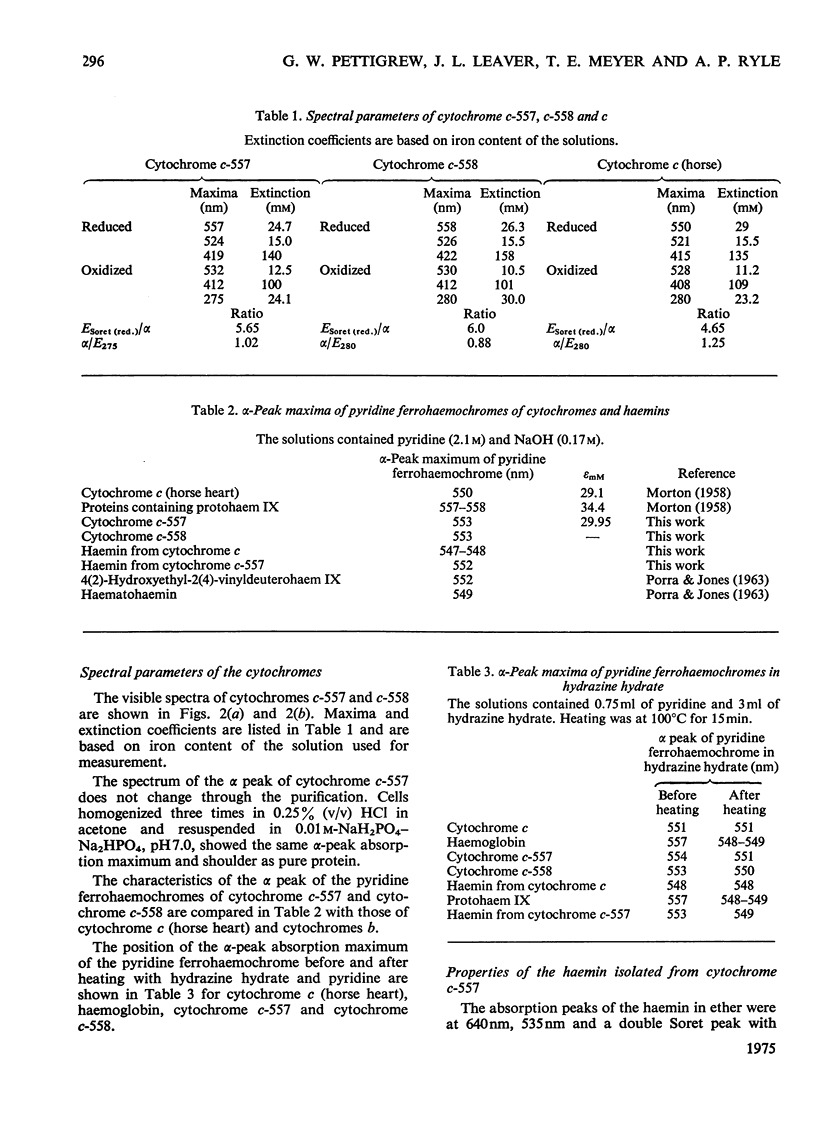
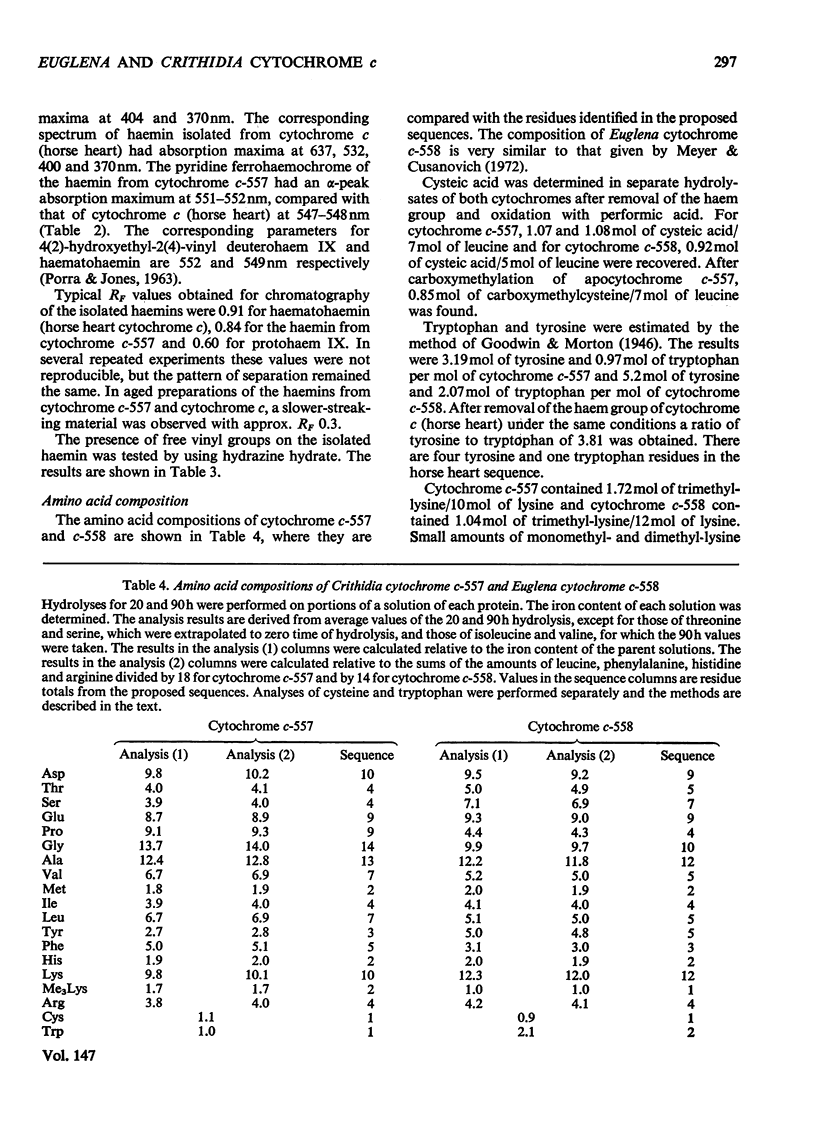
A basic cytochrome was isolated from the phytomastigophorean protozoan Euglena gracilis and a similar protein from the zoomastigophorean protozoan Crithidia oncopelti. In both cases chromatography on CM-cellulose in first the reduced and then the oxidized form proved to be an efficient means of purification. The two cytochromes can be classed in the cytochrome c family but they have certain atypical features. The alpha peak of the absorption spectrum is shifted towards the red and is asymmetrical. The pyridine ferrohaemochrome has an alpha-peak maximum intermediate between that of c-type cytochromes and proteins containing protohaem IX. The test for free vinyl groups was positive. The amino acid sequences of the two cytochromes were determined. Attention is drawn in the text to those parts of the evidence that are less satisfactory. Both sequences are homologous with the family of cytochrome c, but are unusual in having only one cysteine residue so that the haem is attached through only one thioether bond. Detailed evidence for the amino acid dequences of the two proteins has been deposited as Supplementary Publication SUP 50042 (70 pages) at the British Library (Lending Division) (formerly the National Lending Library for Science and Technology), Boston Spa, Wetherby, Yorks. LS23 7BQ, U.K., from whom copies can be obtained on the terms indicated in Biochem. J. (1975) 145, 5.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P., REES M. W. Epsilon-N-Methyl-lysine in bacterial flagellar protein. Nature. 1959 Jul 4;184:56–57. doi: 10.1038/184056b0. [DOI] [PubMed] [Google Scholar]
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Brown L. H. The amino acid sequence of Pseudomonas fluorescens azurin. Biochem J. 1967 Sep;104(3):784–825. doi: 10.1042/bj1040784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BREITENBACH J. W., DERKOSCH J., WESSELY F. Energetics of peptide formation. Nature. 1952 May 31;169(4309):922–922. doi: 10.1038/169922a0. [DOI] [PubMed] [Google Scholar]
- CHU T. C., CHU E. J. H. Paper chromatography of iron complexes of porphyrins. J Biol Chem. 1955 Jan;212(1):1–7. [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Cameron B. F. Determination of iron in heme compounds. II. Hemoglobin and myoglobin. Anal Biochem. 1965 May;11(2):164–169. doi: 10.1016/0003-2697(65)90002-3. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Trager W. Nutritional significance of symbiotic bacteria in two species of hemoflagellates. Science. 1974 Feb 8;183(4124):531–532. doi: 10.1126/science.183.4124.531. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Davis K. A., Hatefi Y., Salemme F. R., Kamen M. D. Enzymic redox reactions of cytochromes c. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1329–1335. doi: 10.1016/0006-291x(72)90612-2. [DOI] [PubMed] [Google Scholar]
- DeLange R. J., Glazer A. N., Smith E. L. Presence and location of an unusual amino acid, epsilon-N-trimethyllysine, in cytochrome c of wheat germ and Neurospora. J Biol Chem. 1969 Mar 10;244(5):1385–1388. [PubMed] [Google Scholar]
- Dixon H. B., Thompson C. M. Chromatography of oxidized and reduced cytochrome c on carboxymethylcellulose. Biochem J. 1968 Apr;107(3):427–431. doi: 10.1042/bj1070427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Rueckert R. R. Observations on molecular weight determinations on polyacrylamide gel. J Biol Chem. 1969 Sep 25;244(18):5074–5080. [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill G. C., Chan S. K., Smith L. Purification and properties of cytochrome c 555 from a protozoan, Crithidia fasciculata. Biochim Biophys Acta. 1971 Nov 2;253(1):78–87. doi: 10.1016/0005-2728(71)90235-0. [DOI] [PubMed] [Google Scholar]
- Kamen M. D., Horio T. Bacterial cytochromes. I. Structural aspects. Annu Rev Biochem. 1970;39:673–700. doi: 10.1146/annurev.bi.39.070170.003325. [DOI] [PubMed] [Google Scholar]
- Keller R. M., Pettigrew G. W., Wüthrich K. Structural studies by proton NMR of cytochrome C-557 from Crithidia oncopelti. FEBS Lett. 1973 Oct 15;36(2):151–156. doi: 10.1016/0014-5793(73)80358-8. [DOI] [PubMed] [Google Scholar]
- Kusel J. P., Suriano J. R., Weber M. M. Isolation, purification, and characterization of Crithidia fasciculata cytochrome c555. Arch Biochem Biophys. 1969 Sep;133(2):293–304. doi: 10.1016/0003-9861(69)90457-3. [DOI] [PubMed] [Google Scholar]
- Lin D. M., Niece R. L., Fitch W. M. The properties and amino-acid sequence of cytochrome c from Euglena gracilis. Nature. 1973 Feb 23;241(5391):533–535. doi: 10.1038/241533a0. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E. Amino acid sequence of chymotryptic peptides from horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2161–2174. [PubMed] [Google Scholar]
- MARGOLIASH E., KIMMEL J. R., HILL R. L., SCHMIDT W. R. Amino acid composition of horse heart cytochrome c. J Biol Chem. 1962 Jul;237:2148–2150. [PubMed] [Google Scholar]
- Margoliash E., Schejter A. Cytochrome c. Adv Protein Chem. 1966;21:113–286. doi: 10.1016/s0065-3233(08)60128-x. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Cusanovich M. A. Euglena gracilis cytochrome 558. Biochim Biophys Acta. 1972 May 25;267(2):383–387. doi: 10.1016/0005-2728(72)90125-9. [DOI] [PubMed] [Google Scholar]
- Newton B. A. Biochemical peculiarities of trypanosomatid flagellates. Annu Rev Microbiol. 1968;22:109–130. doi: 10.1146/annurev.mi.22.100168.000545. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- PERINI F., KAMEN M. D., SCHIFF J. A. IRON-CONTAINING PROTEINS IN EUGLENA. I. DETECTION AND CHARACTERIZATION. Biochim Biophys Acta. 1964 Jul 29;88:74–90. doi: 10.1016/0926-6577(64)90155-x. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W. The amino acid sequence of a cytochrome c from a protozoan Crithidia oncopelti. FEBS Lett. 1972 Apr 15;22(1):64–66. doi: 10.1016/0014-5793(72)80220-5. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W. The amino-acid sequence of cytochrome c from Euglena gracilis. Nature. 1973 Feb 23;241(5391):531–533. doi: 10.1038/241531a0. [DOI] [PubMed] [Google Scholar]
- Pettigrew G. W. The purification and amino acid sequence of cytochrome C-552 from Euglena gracilis. Biochem J. 1974 May;139(2):449–459. doi: 10.1042/bj1390449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramshaw J. A., Thompson E. W., Boulter D. The amino acid sequence of Helianthus annuus L. (sunflower) cyrochrome c deduced from chymotryptic peptides. Biochem J. 1970 Sep;119(3):535–539. doi: 10.1042/bj1190535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmer G., Kreil G. Micro method for detection of formyl and acetyl groups in proteins. Anal Biochem. 1969 May;29(2):186–192. doi: 10.1016/0003-2697(69)90301-7. [DOI] [PubMed] [Google Scholar]
- Sletten K., Dus K., De Klerk H., Kamen M. D. Cytochrome c2 of Rhodospirillum rubrum. 1. Molecular properties of the protein and amino acid sequences of its peptides derived by the action of trypsin and thermolysin. J Biol Chem. 1968 Oct 25;243(20):5492–5506. [PubMed] [Google Scholar]
- Thompson E. W., Notton B. A., Richardson M., Boulter D. The amino acid sequence of cytochrome c from Abutilon theophrasti Medic. and Gossypium barbadense L. (cotton). Biochem J. 1971 Oct;124(4):787–791. doi: 10.1042/bj1240787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner H., White W. N., Hoare D. G., Koshland D. E., Jr The formation of anhydrochymotrypsin by removing the elements of water from the serine at the active site. J Am Chem Soc. 1966 Aug 20;88(16):3851–3859. doi: 10.1021/ja00968a033. [DOI] [PubMed] [Google Scholar]


