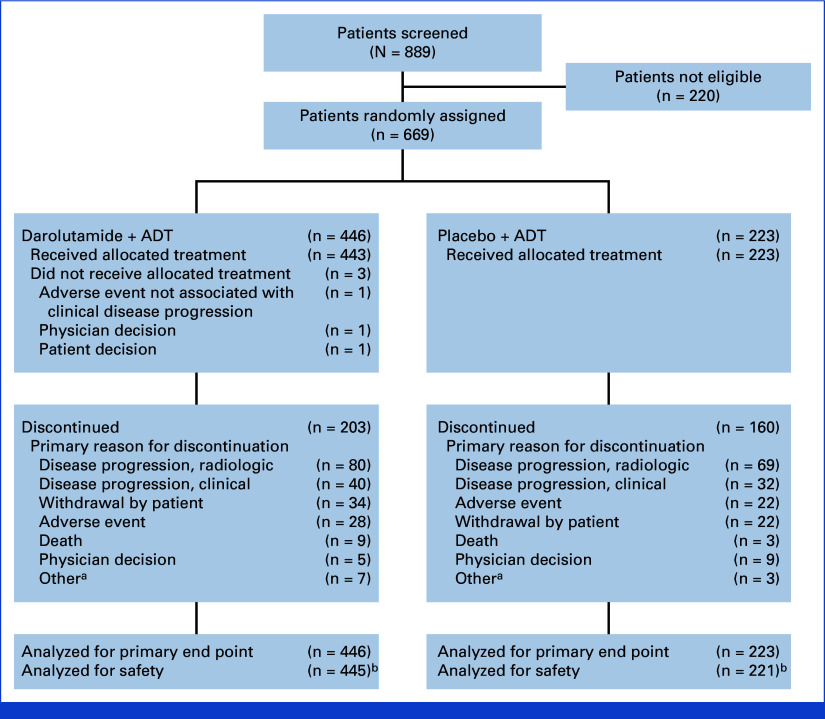
FIG 1.
CONSORT flow diagram. aOther includes required study drug interruption longer than allowed per protocol, additional primary malignancy, noncompliance with study drug, loss to follow-up, and unspecified other reason. bTwo patients who were randomly assigned to the placebo group but received darolutamide are analyzed in the darolutamide group for the safety analysis set. ADT, androgen deprivation therapy.