Abstract
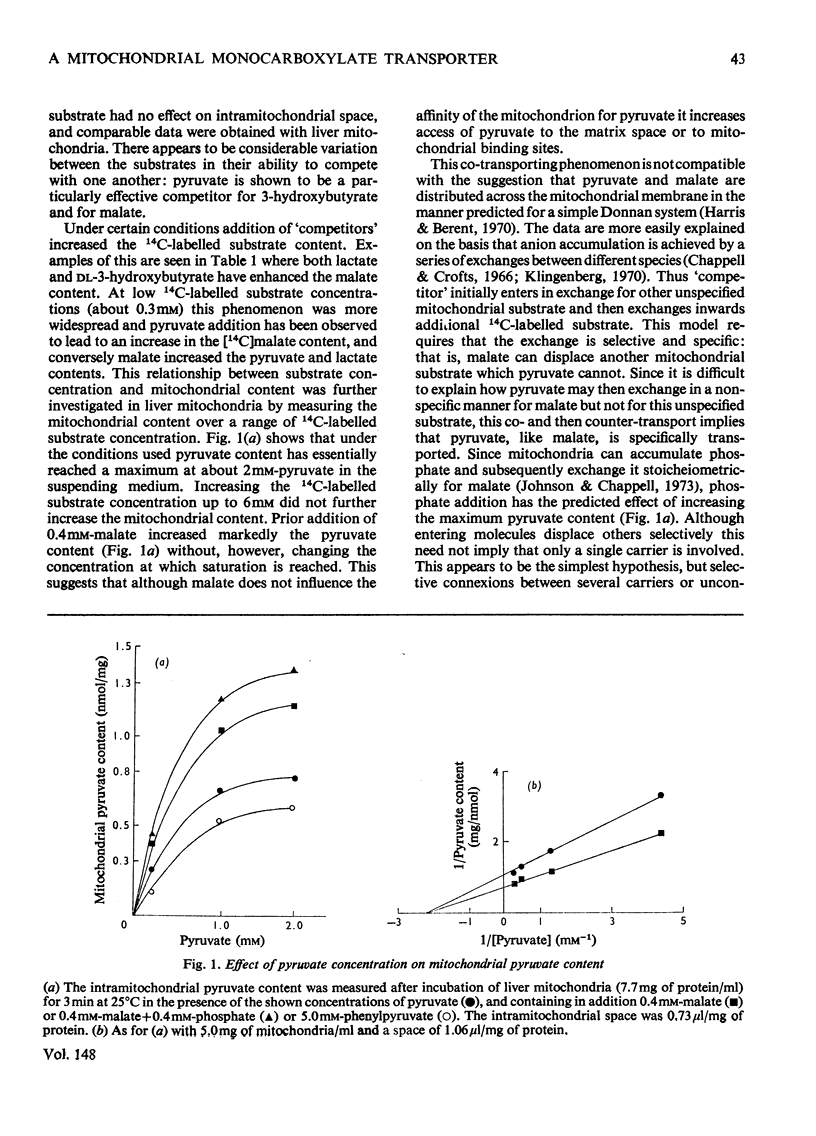
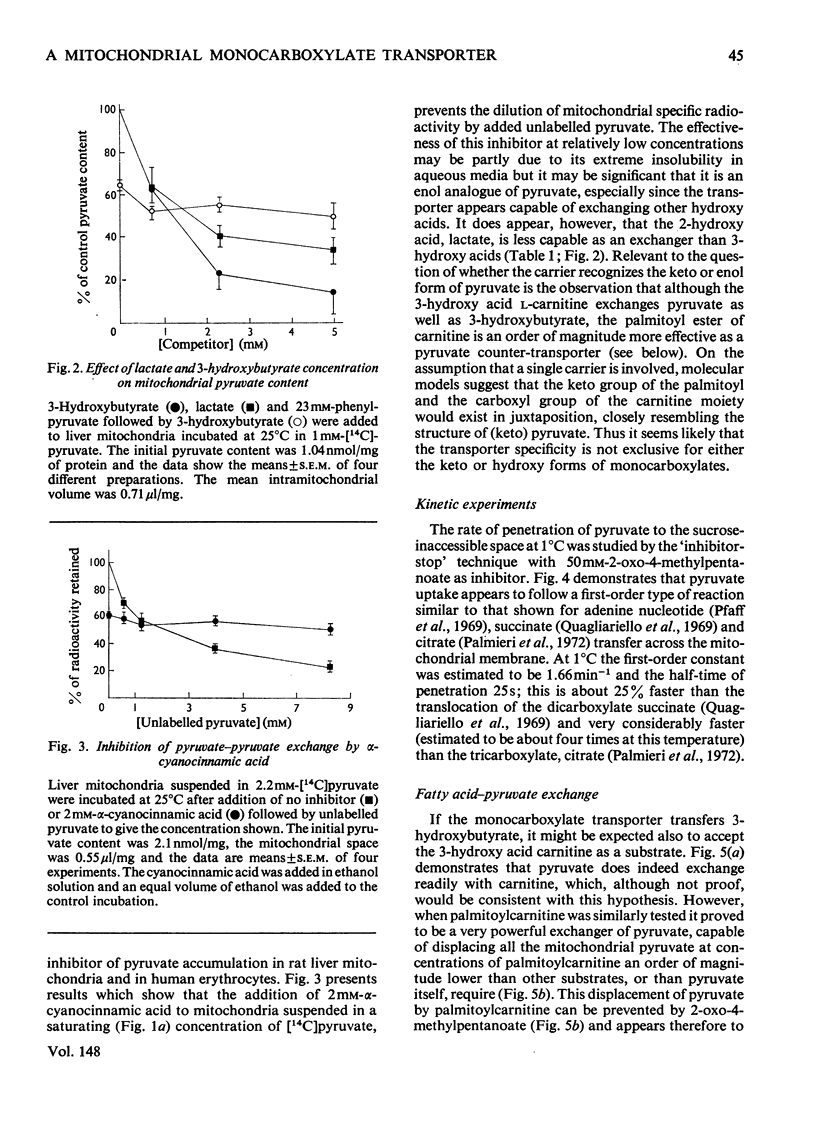
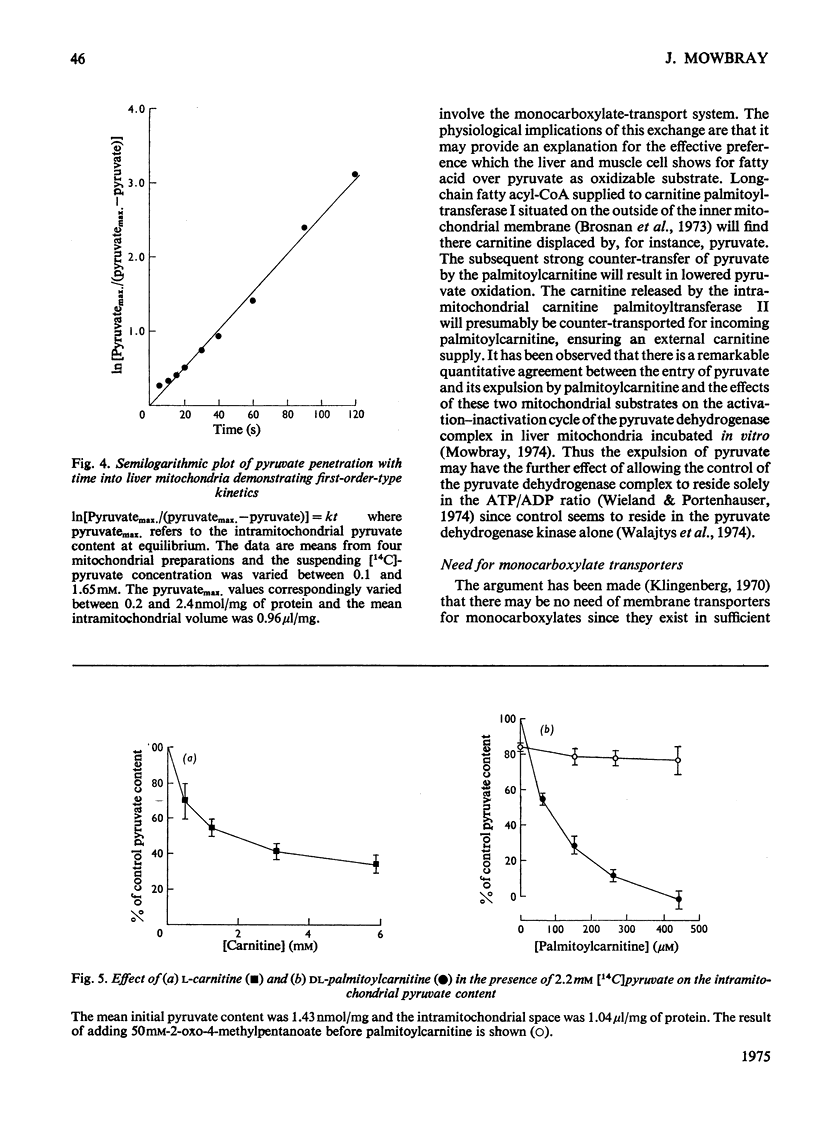
Several hydroxy- and keto-substituted monocarboxylates were found to undergo co- as well as counter-exchange across the mitochondrial membrane. The results argue against a simple Donnan system and may be explained by the existence of a transporter for monocarboxylates. In support of this explanation it was apparently possible to 'pump' pyruvate to the sucrose-inaccessible space by using the dicarboxylate transporter. Further, several aromatic and aliphatic analogues of pyruvate, but not of di- or tri-carboxylate transport inhibitors, have been shown to prevent pyruvate-exchange reactions. Palmitoylcarnitine was found to have a much stronger affinity for the carrier than either carnitine or pyruvate and the possible consequences of this for carnitine-palmitoylcarnitine exchange and on the control of the pyruvate dehydrogenase complex are explored. In view of the range of transport inhibitors and substrates it is suggested that the carrier has a fairly broad specificity. 'Inhibitor-stop' kinetic studies show that the speed of translocation of pyruvate at 1 degrees C is of the same order as malate. The possible correlation between the role of a hydroxy-keto acid transporter in substrate exchange and some whole animal experiments is briefly discussed. It is proposed that for reasons of control the cell will require membrane monocarboxylate transporters no less than di- or tri-carboxylate carriers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arinze I. J., Patel M. S. Inhibition by phenylpyruvate of gluconeogenesis in the isolated perfused rat liver. Biochemistry. 1973 Oct 23;12(22):4473–4479. doi: 10.1021/bi00746a027. [DOI] [PubMed] [Google Scholar]
- Bowden J. A., McArthur C. L., 3rd Possible biochemical model for phenylketonuria. Nature. 1972 Jan 28;235(5335):230–230. doi: 10.1038/235230a0. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Clark J. B., Land J. M. Differential effects of 2-oxo acids on pyruvate utilization and fatty acid synthesis in rat brain. Biochem J. 1974 Apr;140(1):25–29. doi: 10.1042/bj1400025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Specific inhibition of pyruvate transport in rat liver mitochondria and human erythrocytes by alpha-cyano-4-hydroxycinnamate. Biochem J. 1974 Feb;138(2):313–316. doi: 10.1042/bj1380313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Berent Celia. The applicability of the donnan relation to the distribution of certain anions between mitochondria and medium. FEBS Lett. 1970 Sep 18;10(1):6–12. doi: 10.1016/0014-5793(70)80403-3. [DOI] [PubMed] [Google Scholar]
- Harris E. J., Manger J. R. Intramitochondrial substrate concentration as a factor controlling metabolism. The role of interanion competition. Biochem J. 1968 Sep;109(2):239–246. doi: 10.1042/bj1090239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R. N., Chappell J. B. The transport of inorganic phosphate by the mitochondrial dicarboxylate carrier. Biochem J. 1973 Jul;134(3):769–774. doi: 10.1042/bj1340769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Kinetic study of the tricarboxylate carrier in rat liver mitochondria. Eur J Biochem. 1972 Apr 24;26(4):587–594. doi: 10.1111/j.1432-1033.1972.tb01801.x. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Metabolite transport in mitochondria: an example for intracellular membrane function. Essays Biochem. 1970;6:119–159. [PubMed] [Google Scholar]
- Kraupp O., Adler-Kastner L., Niessner H., Plank B. The effects of starvation and of acute and chronic alloxan diabetes on myocardial substrate levels and on liver glycogen in the rat in vivo. Eur J Biochem. 1967 Sep;2(2):197–214. doi: 10.1111/j.1432-1033.1967.tb00126.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Land J. M., Clark J. B. Effect of phenylpyruvate on pyruvate dehydrogenase activity in rat brain mitochondria. Biochem J. 1973 Jun;134(2):539–544. doi: 10.1042/bj1340539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land J. M., Clark J. B. Inhibition of pyruvate and beta-hydroxybutyrate oxidation in rat brain mitochondria by phenylpyruvate and alpha-ketoisocaproate. FEBS Lett. 1974 Aug 30;44(3):348–351. [PubMed] [Google Scholar]
- Mowbray J. Evidence for the role of a specific monocarboxylate transporter in the control of pyruvate oxidation by rat liver mitochondria. FEBS Lett. 1974 Aug 30;44(3):344–347. doi: 10.1016/0014-5793(74)81174-9. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Ottaway J. H. The effect of insulin and growth hormone on the flux of tracer from labelled lactate in perfused rat heart. Eur J Biochem. 1973 Jul 16;36(2):369–379. doi: 10.1111/j.1432-1033.1973.tb02921.x. [DOI] [PubMed] [Google Scholar]
- Mowbray J., Ottaway J. H. The flux of pyruvate in perfused rat heart. Eur J Biochem. 1973 Jul 16;36(2):362–368. doi: 10.1111/j.1432-1033.1973.tb02920.x. [DOI] [PubMed] [Google Scholar]
- Oldendorf W. H. Carrier-mediated blood-brain barrier transport of short-chain monocarboxylic organic acids. Am J Physiol. 1973 Jun;224(6):1450–1453. doi: 10.1152/ajplegacy.1973.224.6.1450. [DOI] [PubMed] [Google Scholar]
- Pfaff E., Heldt H. W., Klingenberg M. Adenine nucleotide translocation of mitochondria. Kinetics of the adenine nucleotide exchange. Eur J Biochem. 1969 Oct;10(3):484–493. doi: 10.1111/j.1432-1033.1969.tb00715.x. [DOI] [PubMed] [Google Scholar]
- Quagliariello E., Palmieri F., Prezioso G., Klingenberg M. Kinetics of succinate uptake by rat-liver mitochondria. FEBS Lett. 1969 Aug;4(4):251–254. doi: 10.1016/0014-5793(69)80247-4. [DOI] [PubMed] [Google Scholar]
- Walajtys E. I., Gottesman D. P., Williamson J. R. Regulation of pyruvate dehydrogenase in rat liver mitochondria by phosphorylation-dephosphorylation. J Biol Chem. 1974 Mar 25;249(6):1857–1865. [PubMed] [Google Scholar]
- Watts D. J., Randle P. J. Evidence for the existence of a pyruvate permease in rat-heart muscle. Biochem J. 1967 Sep;104(3):51P–51P. [PMC free article] [PubMed] [Google Scholar]
- Wieland O. H., Portenhauser R. Regulation of pyruvate-dehydrogenase interconversion in rat-liver mitochondria as related to the phosphorylation state of intramitochondrial adenine nucleotides. Eur J Biochem. 1974 Jun 15;45(2):577–588. doi: 10.1111/j.1432-1033.1974.tb03584.x. [DOI] [PubMed] [Google Scholar]
- Williamson J. R. Effects of insulin and starvation on the metabolism of acetate and pyruvate by the perfused rat heart. Biochem J. 1964 Oct;93(1):97–106. doi: 10.1042/bj0930097. [DOI] [PMC free article] [PubMed] [Google Scholar]


