Fig. 3 ∣. Controlling the chemical environment in high-throughput evolution.
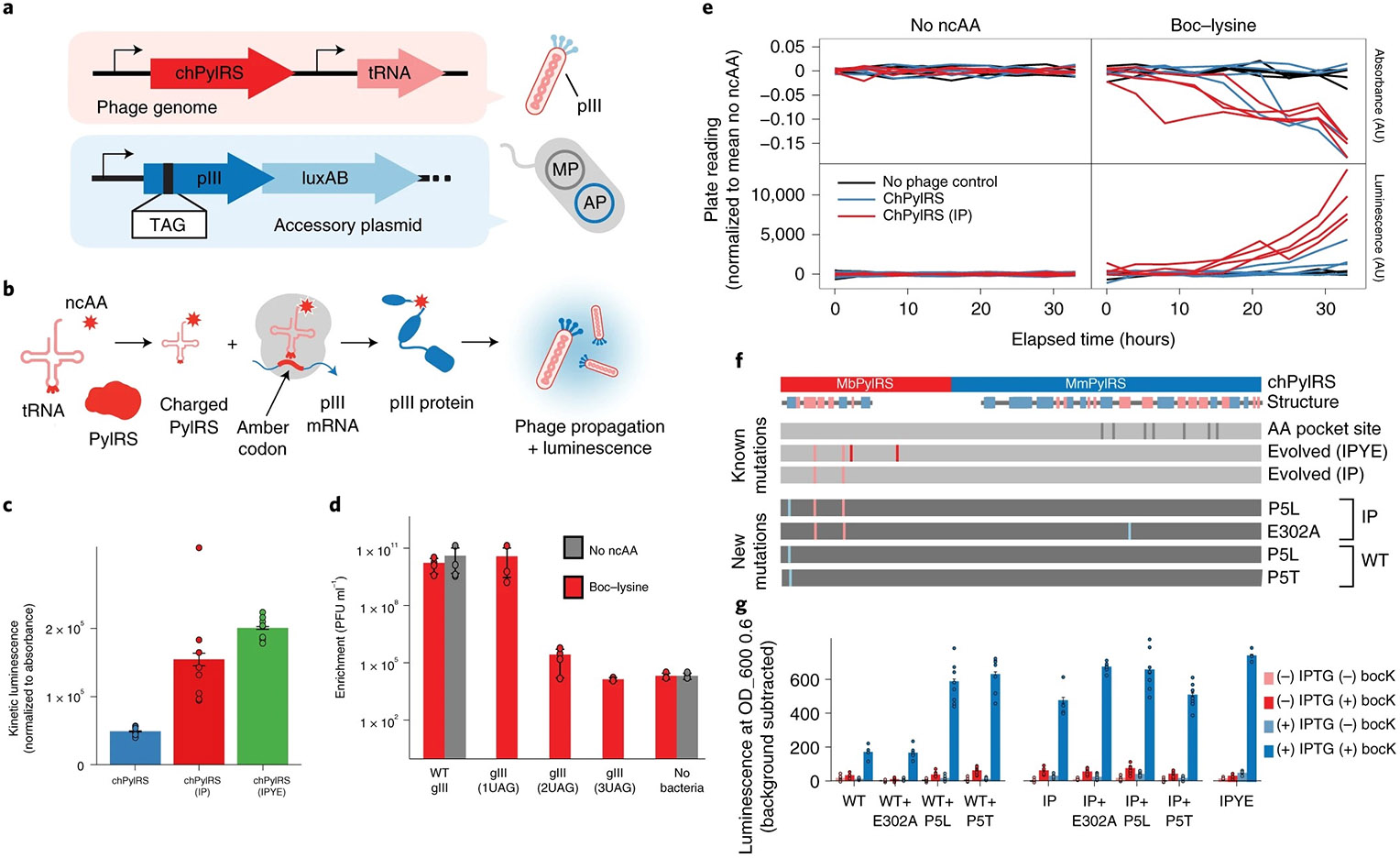
a, Strategy for evolving AARSs and tRNAs to incorporate noncanonical amino acids. TAG amber codons are inserted into the pIII protein, and the evolving tRNAPyl and chPylRS are encoded in the evolving phage genome. b, Phage propagation and luminescence are contingent on the successful incorporation of ncAAs into pIII. c, Efficiency of unevolved versus evolved chPylRS variants (IP, IPYE mutations) at incorporating Boc-lysine into an inducible TAG-luxAB reporter, normalized to no-ncAA and no-IPTG controls. Data are presented as mean values ± s.e.m. for n = 8 biologically independent samples. d, Quantifying the selection stringency of incorporating one, two or three ncAAs into pIII, in either the presence or absence of Boc-lysine, using the evolved variant chPylRS-IPYE. Data are presented as mean values ± s.e.m. for n = 4 biologically independent samples. e, Real-time absorbance depression and luminescence monitoring beginning from either the unevolved state (chPylRS, blue) or an intermediate evolved state (chPylRS-IP, red). f, Genotypes of the evolved variants. Phage persisted in all of the chPylRS-IP populations, and clonal phage acquired novel mutations (P5L or E302A). Only half of the chPylRS populations resulted in persistent phage propagation at 36 h; each acquired a distinct mutation at the same N-terminal proline residue (P5L or P5T) within the conserved essential N-terminal domain of PylRS44,45. g, Efficiency of evolved variant mutations in both chPylRS and chPylRS-IP at incorporating Boc-lysine into an inducible TAG-luxAB reporter, compared to no-ncAA and no-IPTG controls. Data are presented as mean values ± s.e.m. for n = 8 biologically independent samples.
