Abstract
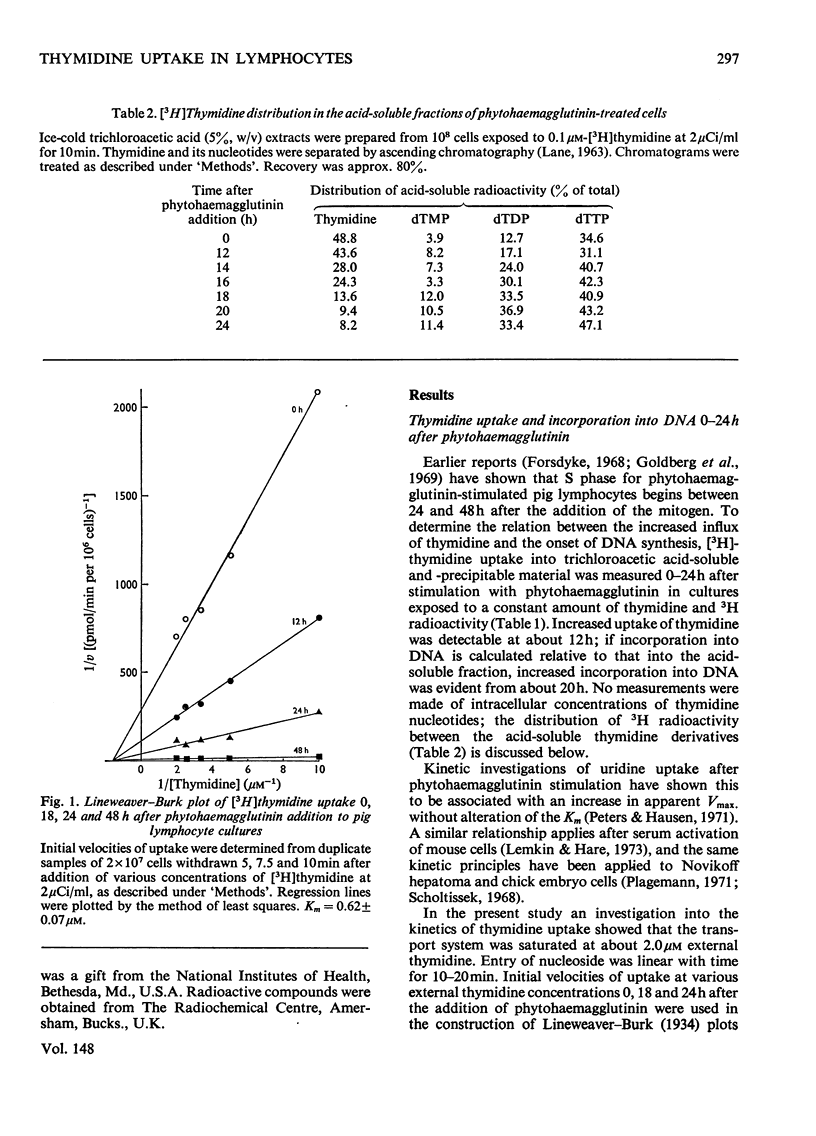
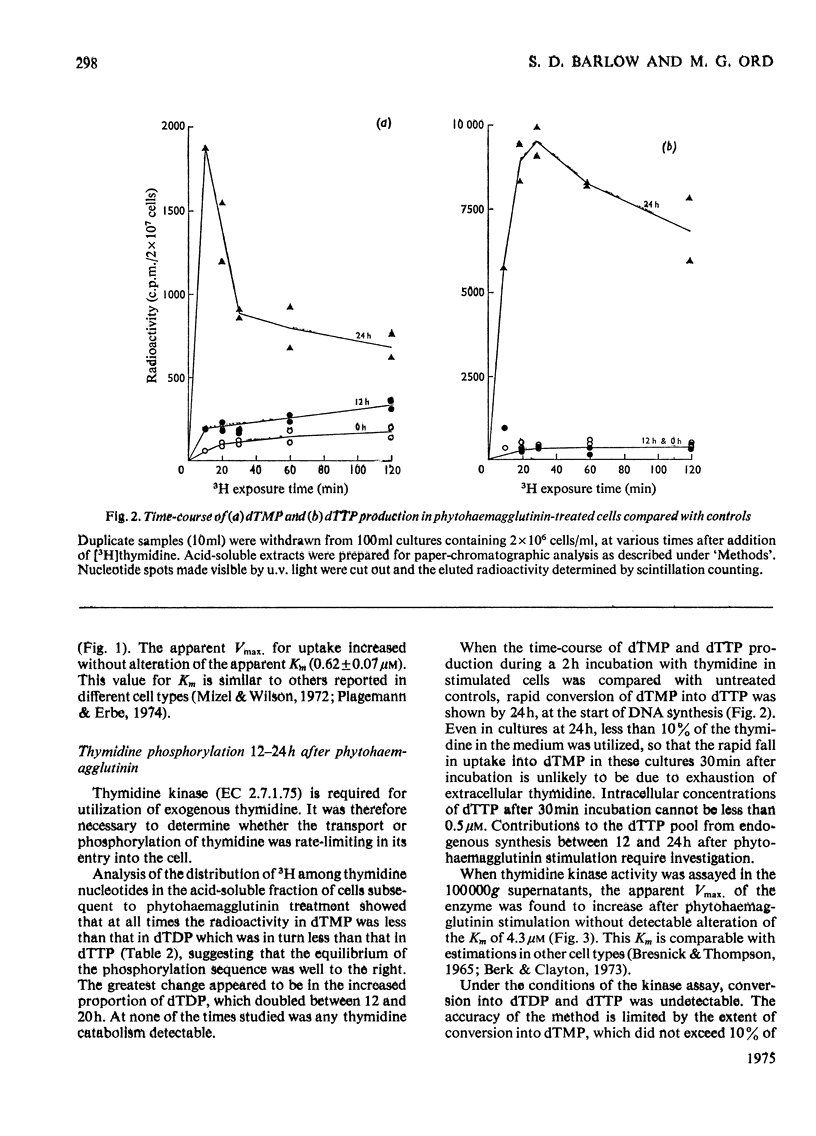
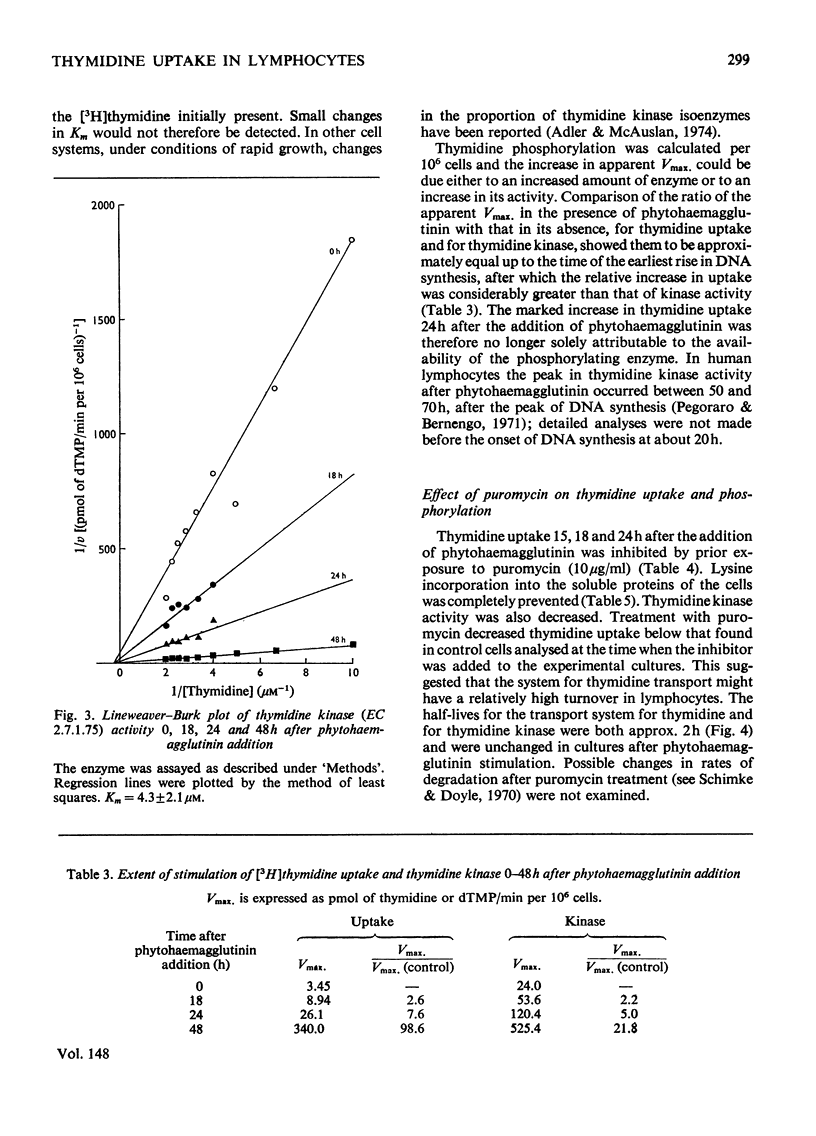
Thymidine and uridine transporters in peripheral pig lymphocytes have structural features in common, but are not identical. Accelerated entry of [3H]thymidine begins 12h after the addition of phytohaemagglutinin. The increased thymidine uptake into the cells is characterized by an increase in Vmax. Without alteration of the apparent Km(0.6+/-0.08muM). Thymidine kinase activity is increased 12h after stimulation. Both the increased thymidine uptake and the increased thymidine kinase activity are inhibited in cultures incubated with puromycin: rates of degradation of the two systems are unchanged after phytohaemagglutinin addition, and indicate similar half-lives of about 2h. Thymidine kinase is rate-limiting for thymidine entry up to 18h after phytohaemagglutinin addition; increase in its synthesis is detectable about 6h before net incorporation of thymidine into DNA is significantly promoted.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler R., McAuslan B. R. Expression of thymidine kinase variants is a function of the replicative state of cells. Cell. 1974 Jun;2(2):113–117. doi: 10.1016/0092-8674(74)90100-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. A genetically distinct thymidine kinase in mammalian mitochondria. Exclusive labeling of mitochondrial deoxyribonucleic acid. J Biol Chem. 1973 Apr 25;248(8):2722–2729. [PubMed] [Google Scholar]
- Bresnick E., Thompson U. B. Properties of deoxythymidine kinase partially purified from animal tumors. J Biol Chem. 1965 Oct;240(10):3967–3974. [PubMed] [Google Scholar]
- Cass C. E., Paterson A. R. Mediated transport of nucleosides in human erythrocytes. Accelerative exchange diffusion of uridine and thymidine and specificity toward pyrimidine nucleosides as permeants. J Biol Chem. 1972 May 25;247(10):3314–3320. [PubMed] [Google Scholar]
- Everhart L. P., Jr, Rubin R. W. Cyclic changes in the cell surface. I. Change in thymidine transport and its inhibition by cytochalasin B in Chinese hamster ovary cells. J Cell Biol. 1974 Feb;60(2):434–441. doi: 10.1083/jcb.60.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALLON H. J., FREI E., 3rd, DAVIDSON J. D., TRIER J. S., BURK D. Leukocyte preparations from human blood: evaluation of their morphologic and metabolic state. J Lab Clin Med. 1962 May;59:779–791. [PubMed] [Google Scholar]
- Forsdyke D. R. Studies on the incorporation of [5-3H] uridine during activation and transformation of lymphocytes induced by phytohaemagglutinin. Dependence on the incorporation rate on uridine concentration at certain critical concentrations. Biochem J. 1968 Mar;107(2):197–205. doi: 10.1042/bj1070197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Rosenau W., Burke G. C. Fractionation of phytohemagglutinin. I. Purification of the RNA and DNA synthesis-stimulating substances and evidence that they are not proteins. Proc Natl Acad Sci U S A. 1969 Sep;64(1):283–289. doi: 10.1073/pnas.64.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIATT H. H., BOJARSKI T. B. Stimulation of thymidylate kinase activity in rat tissues by thymidine administration. Biochem Biophys Res Commun. 1960 Jan;2:35–39. doi: 10.1016/0006-291x(60)90260-6. [DOI] [PubMed] [Google Scholar]
- HURLBERT R. B., SCHMITZ H., BRUMM A. F., POTTER V. R. Nucleotide metabolism. II. Chromatographic separation of acid-soluble nucleotides. J Biol Chem. 1954 Jul;209(1):23–39. [PubMed] [Google Scholar]
- Hare J. D. Quantitative aspects of thymidine uptake into the acid-souble pool of normal and polyoma-transformed hamster cells. Cancer Res. 1970 Mar;30(3):684–691. [PubMed] [Google Scholar]
- Hausen P., Stein H. On the synthesis of RNA in lymphocytes stimulated by phytohemagglutinin. 1. Induction of uridine-kinase and the conversion of uridine to UTP. Eur J Biochem. 1968 Apr;4(3):401–406. doi: 10.1111/j.1432-1033.1968.tb00226.x. [DOI] [PubMed] [Google Scholar]
- IVES D. H., MORSE P. A., Jr, POTTER V. R. Feedback inhibition of thymodine kinase by thymodine triphosphate. J Biol Chem. 1963 Apr;238:1467–1474. [PubMed] [Google Scholar]
- Kay J. E. Early effects of phytohaemagglutinin on lymphocyte RNA synthesis. Eur J Biochem. 1968 Apr 3;4(2):225–232. doi: 10.1111/j.1432-1033.1968.tb00198.x. [DOI] [PubMed] [Google Scholar]
- Kay J. E., Handmaker S. D. Uridine incorporation and RNA synthesis during stimulation of lymphocytes by PHA. Exp Cell Res. 1970 Dec;63(2):411–421. doi: 10.1016/0014-4827(70)90230-2. [DOI] [PubMed] [Google Scholar]
- LANE B. G. The separation of adenosine, guanosine, cytidine and uridine by one-dimensional filter-paper chromatography. Biochim Biophys Acta. 1963 May 28;72:110–112. [PubMed] [Google Scholar]
- Lemkin J. A., Hare J. D. Nucleoside transport in normal and polyoma-transformed cells: kinetic differences following adenosine and serum or insulin stimulation. Biochim Biophys Acta. 1973 Aug 9;318(1):113–122. [PubMed] [Google Scholar]
- Mizel S. B., Wilson L. Nucleoside transport in mammalian cells. Inhibition by colchicine. Biochemistry. 1972 Jul 4;11(14):2573–2578. doi: 10.1021/bi00764a003. [DOI] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Thymidine metabolism in regenerating rat liver one to two hours after practical hepatectomy. Biochem J. 1973 Nov;136(3):571–577. doi: 10.1042/bj1360571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegoraro L., Bernengo M. G. Thymidine kinase, deoxycytidine kinase and deoxycytidylate deaminase activities in phytohaemagglutinin stimulated human lymphocytes. Exp Cell Res. 1971 Oct;68(2):283–290. doi: 10.1016/0014-4827(71)90152-2. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Hausen P. Effect of phytohemagglutinin on lymphocyte membrane transport. I. Stimulation of uridine uptake. Eur J Biochem. 1971 Apr 30;19(4):502–508. doi: 10.1111/j.1432-1033.1971.tb01341.x. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Erbe J. The deoxyribonucleoside transport systems of cultured Novikoff rat hepatoma cells. J Cell Physiol. 1974 Jun;83(3):337–343. doi: 10.1002/jcp.1040830303. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G. Nucleoside transport by Novikoff rat hepatoma cells glowing in suspension culture. Specificity and mechanism of transport reactions and relationship to nucleoside incorporation into nucleic acids. Biochim Biophys Acta. 1971 Jun 1;233(3):688–701. doi: 10.1016/0005-2736(71)90168-4. [DOI] [PubMed] [Google Scholar]
- Plagemann P. G., Richey D. P., Zylka J. M., Erbe J. Thymidine transport by Novikoff rat hepatoma cells synchronized by double hydroxyurea treatment. Exp Cell Res. 1974 Feb;83(2):303–310. doi: 10.1016/0014-4827(74)90343-7. [DOI] [PubMed] [Google Scholar]
- Schimke R. T., Doyle D. Control of enzyme levels in animal tissues. Annu Rev Biochem. 1970;39:929–976. doi: 10.1146/annurev.bi.39.070170.004433. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. Studies on the uptake of nucleic acid precursors into cells in tissue culture. Biochim Biophys Acta. 1968 Jun 24;158(3):435–447. doi: 10.1016/0304-4165(68)90297-3. [DOI] [PubMed] [Google Scholar]
- Schuster G. S., Hare J. D. The role of phosphorylation in the uptake of thymidine in mammalian cells. In Vitro. 1971 May-Jun;6(6):427–436. doi: 10.1007/BF02616044. [DOI] [PubMed] [Google Scholar]


