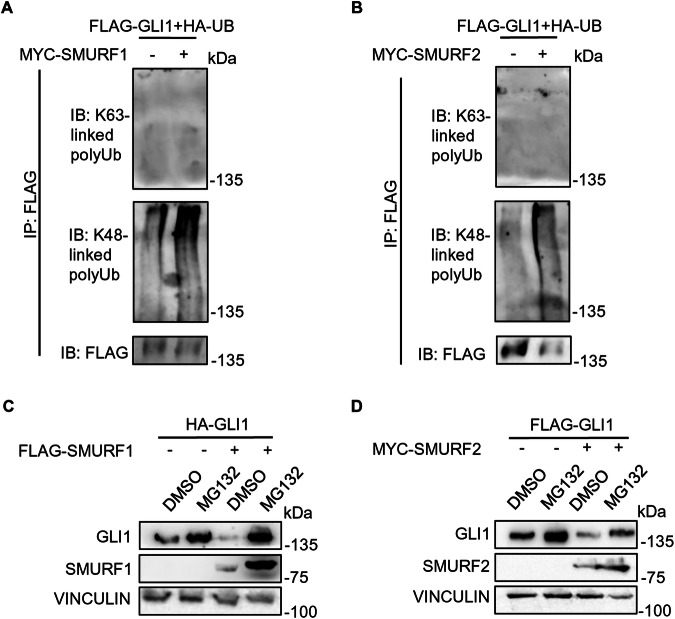
Fig. 3. SMURF proteins mediate GLI1 K48-poly-ubiquitination, leading to GLI1 proteasomal degradation.
A, B Ubiquitination assays of HEK-293T cells following expression of FLAG-GLI1, HA-UB and either control vectors, MYC-SMURF1 or MYC-SMURF2. 24 h after transfection, cells lysates were immunoprecipitated with FLAG agarose beads and analysed by SDS-PAGE. Proteins were detected using antibodies anti-FLAG, anti-MYC and specific antibodies against K48-linked/K63-linked poly-ubiquitination to detect the ubiquitination status of GLI1. C, D Analysis of GLI1 protein levels following SMURF1/SMURF2 expression in presence of proteasome inhibitor MG132. HEK-293T were transfected with HA-GLI1 or FLAG-GLI1, in combination with control vector, FLAG-SMURF1 or MYC-SMURF2. 24 h post transfection, cells were treated with DMSO or MG132 at 1 µM for 16 h. Subsequently, cells were lysed and analysed by SDS-PAGE. Proteins were detected using antibodies anti-FLAG, anti-HA, anti-MYC, and anti-VINCULIN (used as a normalizer).