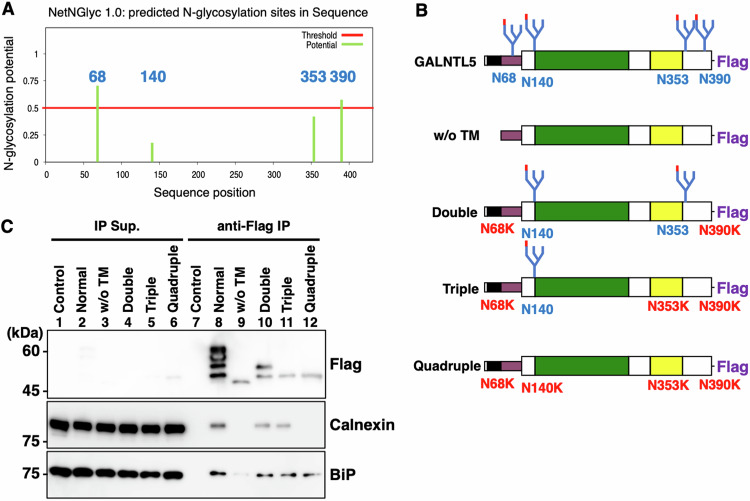
Fig. 3. Interaction of GALNTL5 with the ER-resident proteins calnexin and BiP, the former through ER N-glycosylation.
A A search program for N-glycosylation sites (https://services.healthtech.dtu.dk/services/NetNGlyc-1.0/) predicted that four asparagine residues (68, 140, 353, and 390) in the amino acid sequence of mouse GALNTL5 were N-glycosylation sites. B Schematic comparison of mouse GALNTL5 based on IP experiments. Full-length GALNTL5 contains a transmembrane domain (back), a stem region (purple), and a catalytic unit consisting of a GT1 motif (green), a Gal/GalNAc-T motif (yellow), and a Flag tag at the C-terminus. N68, N140, N353, and N390 indicate asparagine residues that serve as N-glycosylation sites in mouse GALNTL5. “w/o TM” indicates full-length GALNTL5 without the transmembrane domain. The double mutants are characterized by substitution of asparagine for lysine at two amino acid sites (N68K and N390K). The triple mutants show substitution of asparagine for lysine at three amino acid sites (N68K, N353K, and N390K). The quadruple mutants exhibit lysine at all sites (N68K, N140K, N353K, and N390K). C IP with anti-Flag antibody followed by western blotting with anti-Flag antibody. Co-IP with anti-Flag antibody followed by western blotting with anti-calnexin and anti-BiP antibodies.