Abstract
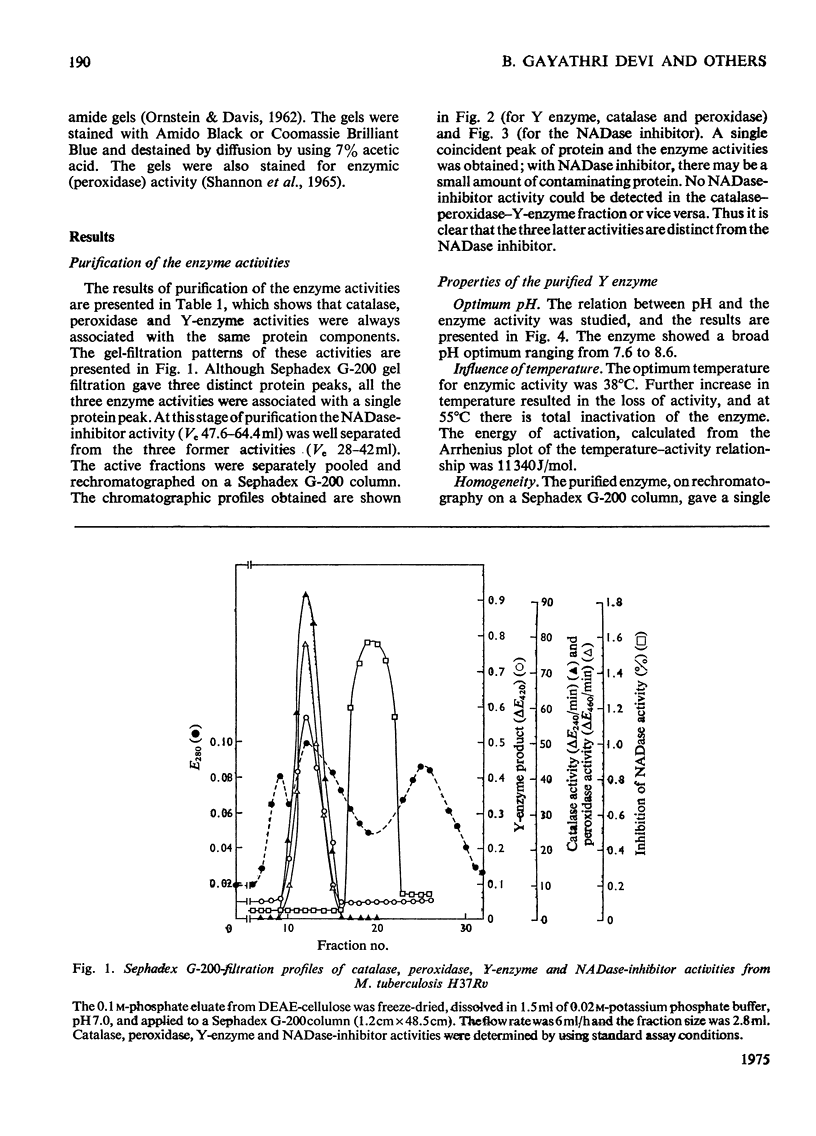
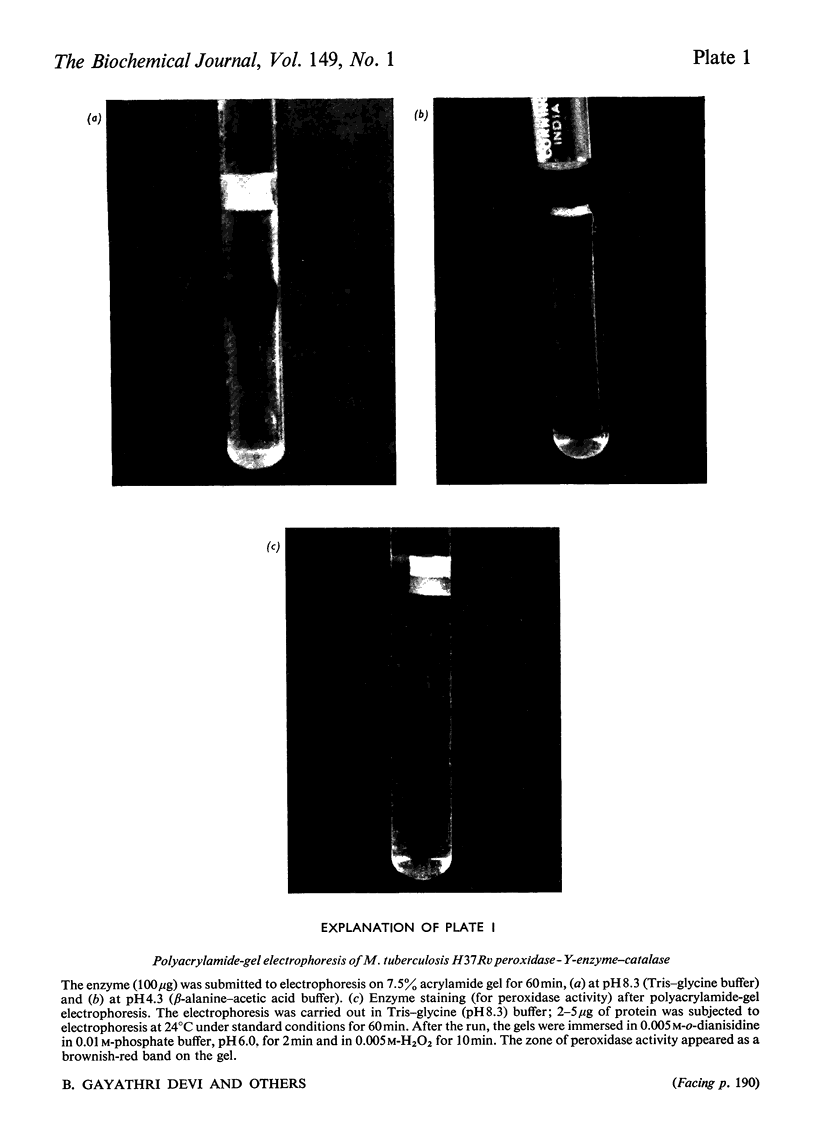
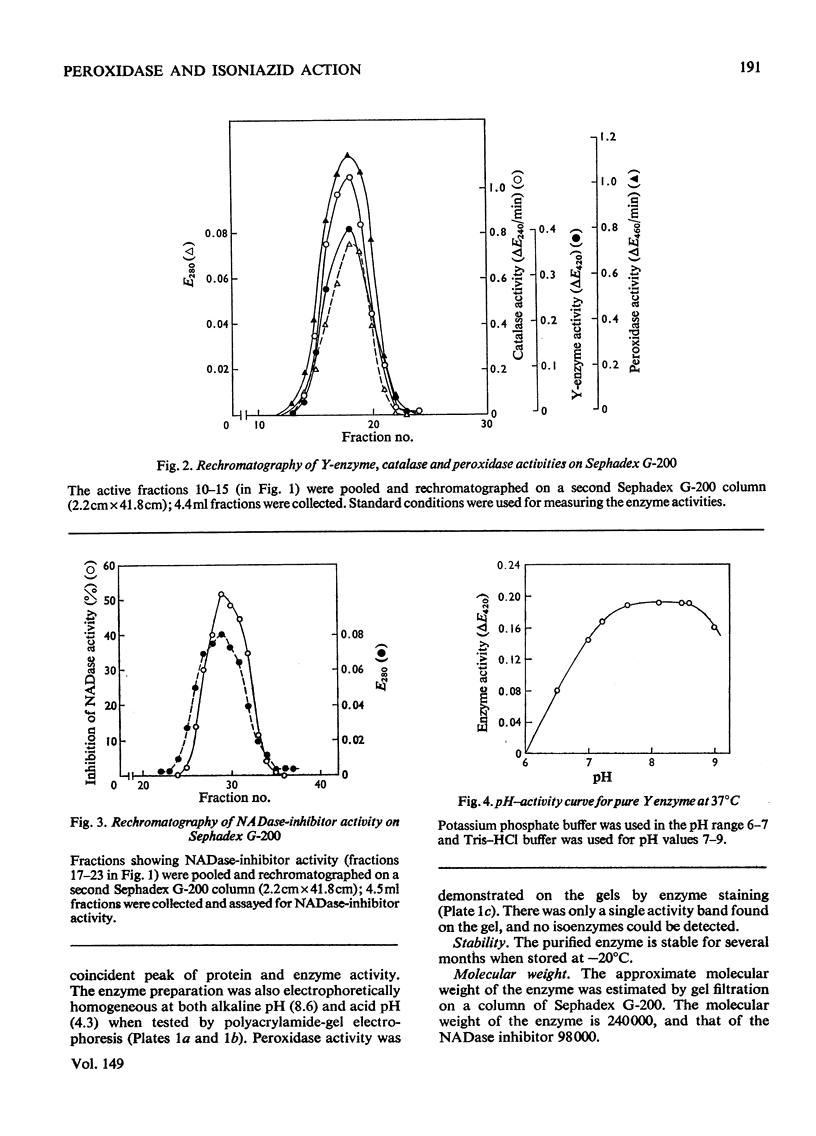
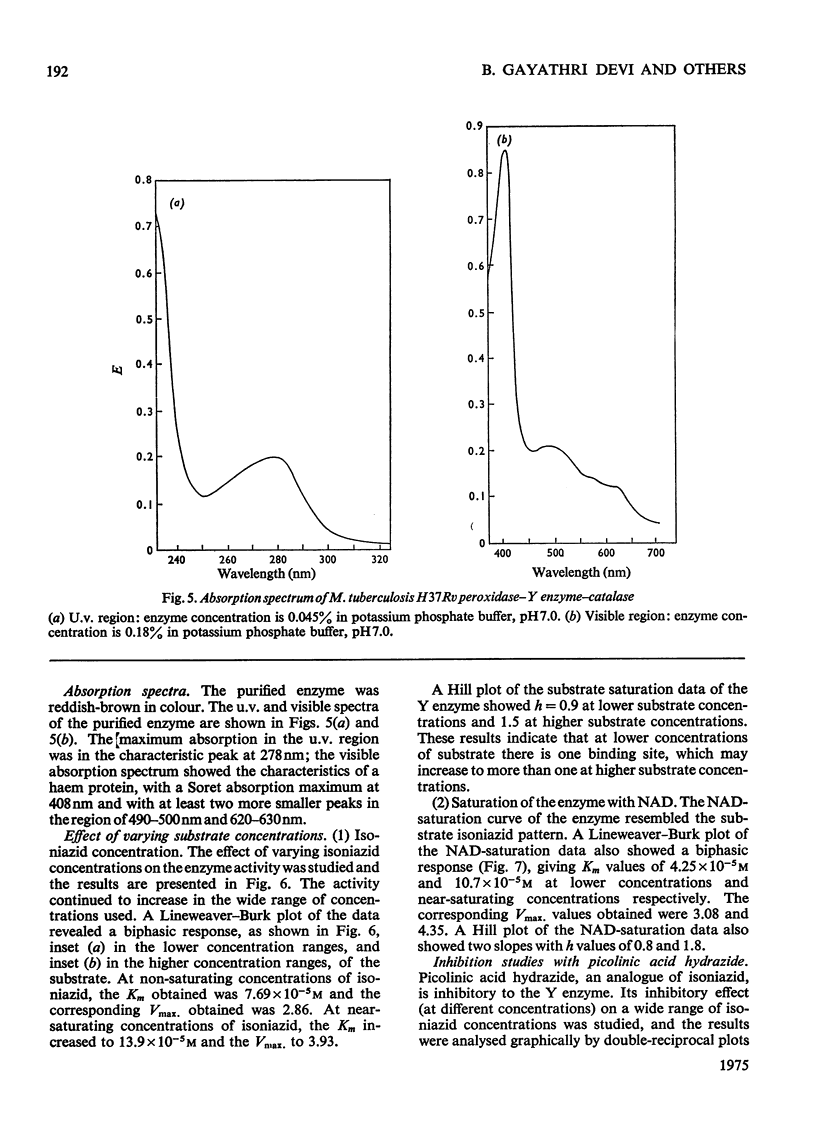
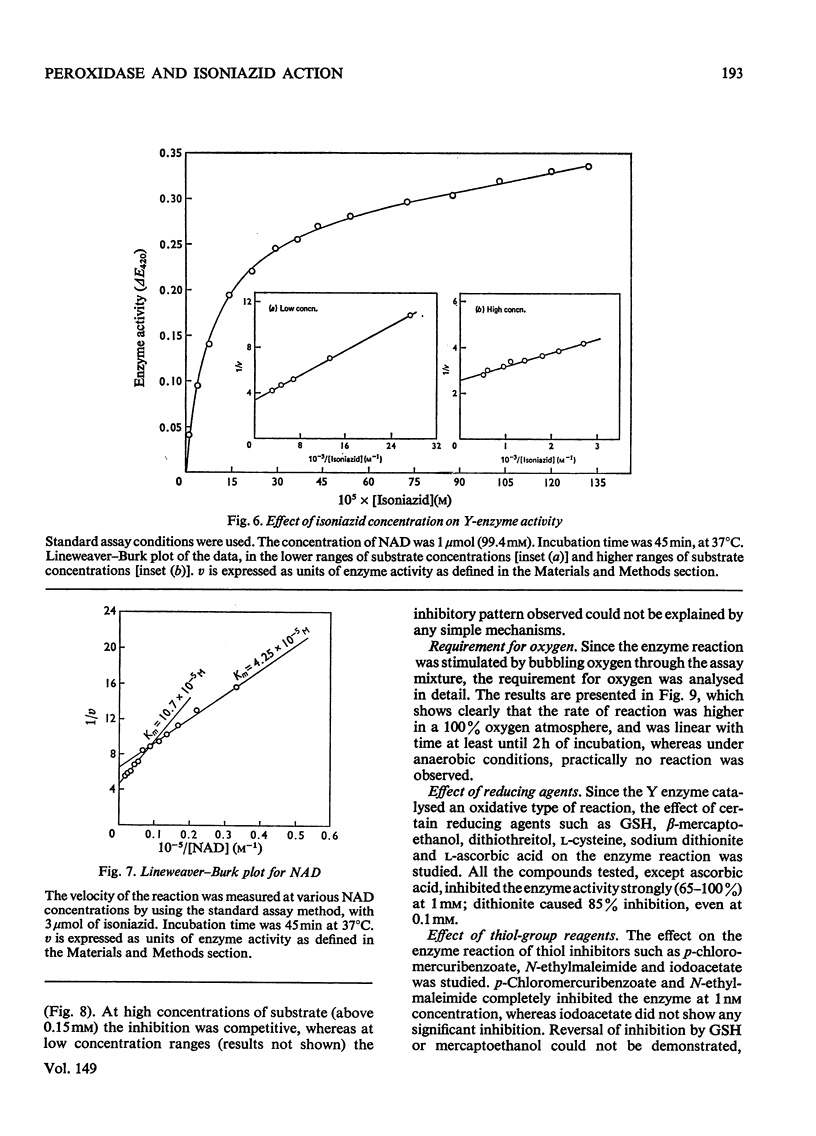
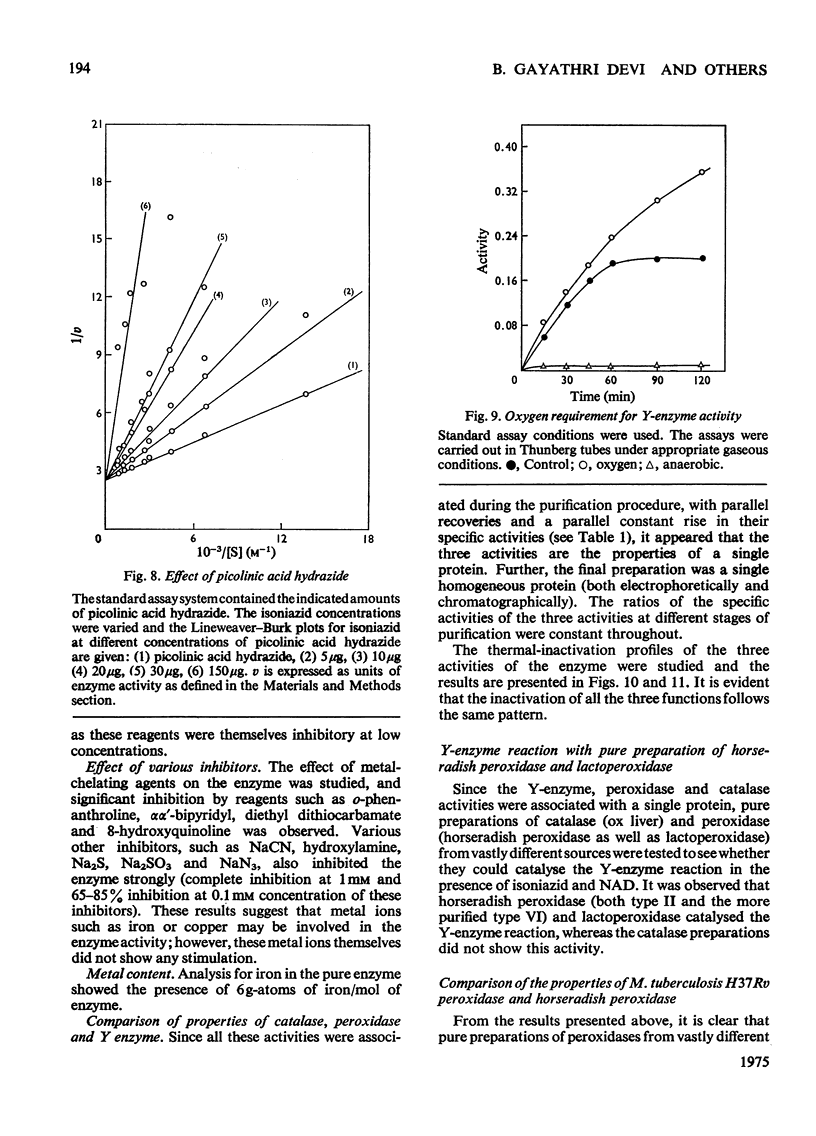
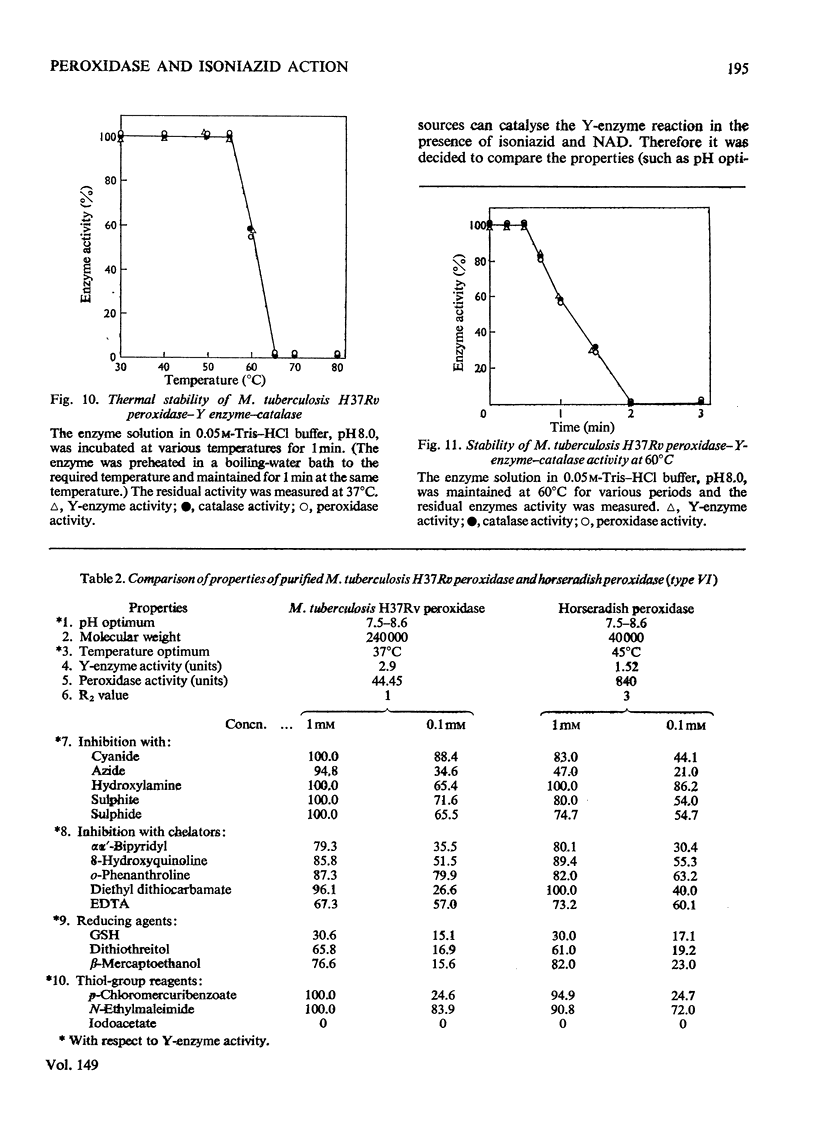
Peroxidase from Mycobacterium tuberculosis H37Rv was purified to homogeneity. The homogeneous protein exhibits catalase and Y (Youatt's)-enzyme activities in addition to peroxidase activity. Further confirmation that the three activities are due to a single enzyme was accomplished by other criteria, such as differential thermal inactivation, sensitivity to different inhibitors, and co-purification. The Y enzyme (peroxidase) was separated from NADase (NAD+ glycohydrolase) inhibitor by gel filtration on Sephadex G-200. The molecular weights of peroxidase and NADase inhibitor, as determined by gel filtration, are 240000 and 98000 respectively. The Y enzyme shows two Km values for both isoniazid (isonicotinic acid hydrazide) and NAD at low and high concentrations. Analysis of the data by Hill plots revealed that the enzyme has one binding site at lower substrate concentrations and more than one at higher substrate concentration. The enzyme contains 6g-atoms of iron/mol. Highly purified preparations of peroxidases from different sources catalyse the Y-enzyme reaction, suggesting that the nature of the reaction may be a peroxidatic oxidation of isoniazid. Moreover, the Y-enzyme reaction is enhanced by O2. Isoniazid-resistant mutants do not exhibit Y-enzyme, peroxidase or catalase activities, and do not take up isoniazid. The Y-enzyme reaction is therefore implicated in the uptake of the drug.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREJEW A., GERNEZ-RIEUX C., TACQUET A. Action de l'INH et de la D-cyclosérine sur la peroxydase purifiée et sur l'activité peroxydasique des bacilles tuberculeux. Ann Inst Pasteur (Paris) 1959 Feb;96(2):145–163. [PubMed] [Google Scholar]
- ANDREJEW A., GERNEZ-RIEUX C., TACQUET A. [Attempts at differentiation of mycobacteria sensitive and resistant to INH by the aid of quantitative and qualitative tests of peroxidase activity. Discussion of technical problems]. Ann Inst Pasteur (Paris) 1960 Dec;99:821–838. [PubMed] [Google Scholar]
- ANDREJEW A., GERNEZRIEUX C., TACQUET A. Activité catalasique des Mycobactéries. Ann Inst Pasteur (Paris) 1956 Nov;91(5):767–770. [PubMed] [Google Scholar]
- Andrejew A., Renard A. Essai de séparation des activités catalasique et peroxydasique chez les Mycobactéries. Ann Inst Pasteur (Paris) 1968 Jul;115(1):3–10. [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bekierkunst A., Bricker A. Studies on the mode of action of isoniazid on mycobacteria. Arch Biochem Biophys. 1967 Nov;122(2):385–392. doi: 10.1016/0003-9861(67)90209-3. [DOI] [PubMed] [Google Scholar]
- COHN M. L., ODA U., KOVITZ C., MIDDLEBROOK G. Studies on isoniazid and tubercle bacilli. I. The isolation of isoniazid-resistant mutants in vitro. Am Rev Tuberc. 1954 Sep;70(3):465–475. doi: 10.1164/art.1954.70.3.465. [DOI] [PubMed] [Google Scholar]
- DARTER R. W., MILLMAN I. Localization of mycobacterial enzymes. Proc Soc Exp Biol Med. 1957 Jul;95(3):440–446. doi: 10.3181/00379727-95-23245. [DOI] [PubMed] [Google Scholar]
- Gopinathan K. P., Ramakrishnan T., Vaidyanathan C. S. Purification and properties of an inhibitor for nicotinamide-adenine dinucleotidase from Mycobacterium tuberculosis H-37-Rv. Arch Biochem Biophys. 1966 Feb;113(2):376–382. doi: 10.1016/0003-9861(66)90201-3. [DOI] [PubMed] [Google Scholar]
- HEDGECOCK L. W., FAUCHER I. O. Relation of pyrogallol-peroxidative activity to isoniazid resistance in Mycobacterium tuberculosis. Am Rev Tuberc. 1957 Apr;75(4):670–674. doi: 10.1164/artpd.1957.75.4.670. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MIDDLEBROOK G., COHN M. L., SCHAEFER W. B. Studies on isoniazid and tubercle bacilli. III. The isolation, drug-susceptibility, and catalase-testing of tubercle bacilli from isoniazid-treated patients. Am Rev Tuberc. 1954 Nov;70(5):852–872. doi: 10.1164/art.1954.70.5.852. [DOI] [PubMed] [Google Scholar]
- MIDDLEBROOK G. Isoniazid-resistance and catalase activity of tubercle bacilli; a preliminary report. Am Rev Tuberc. 1954 Mar;69(3):471–472. doi: 10.1164/art.1954.69.3.471. [DOI] [PubMed] [Google Scholar]
- MIDDLEBROOK G. Sterilization of tubercle bacilli by isonicotinic acid hydrazide and the incidence of variants resistant to the drug in vitro. Am Rev Tuberc. 1952 Jun;65(6):765–767. [PubMed] [Google Scholar]
- SZYBALSKI W., BRYSON V. Bacterial resistance studies with derivatives of isonicotinic acid. Am Rev Tuberc. 1952 Jun;65(6):768–770. [PubMed] [Google Scholar]
- Shannon L. M., Kay E., Lew J. Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J Biol Chem. 1966 May 10;241(9):2166–2172. [PubMed] [Google Scholar]
- Sriprakash K. S., Ramakrishnan T. Isoniazid-resistant mutants of Mycobacterium tuberculosis H37RV: uptake of isoniazid and the properties of NADase inhibitor. J Gen Microbiol. 1970 Jan;60(1):125–132. doi: 10.1099/00221287-60-1-125. [DOI] [PubMed] [Google Scholar]
- TSUKAMURA M. Further mutational studies on the isoniazid resistance of Mycobacterium tuberculosis. Jpn J Microbiol. 1960 Apr;4:115–122. doi: 10.1111/j.1348-0421.1960.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Van de Bogart M., Beinert H. Micro methods for the quantitative determination of iron and copper in biological material. Anal Biochem. 1967 Aug;20(2):325–334. doi: 10.1016/0003-2697(67)90038-3. [DOI] [PubMed] [Google Scholar]
- WINDER F. Catalase and peroxidase in mycobacteria. Possible relationship to the mode of action of isoniazid. Am Rev Respir Dis. 1960 Jan;81:68–78. doi: 10.1164/arrd.1960.81.1P1.68. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W. Effect of isoniazid on biosynthesis in Mycobacterium tuberculosis var. bovis BCG. J Gen Microbiol. 1967 Jun;47(3):379–388. doi: 10.1099/00221287-47-3-379. [DOI] [PubMed] [Google Scholar]
- Wimpenny J. W. The uptake and fate of isoniazid in Mycobacterium tuberculosis var. bovis BCG. J Gen Microbiol. 1967 Jun;47(3):389–403. doi: 10.1099/00221287-47-3-389. [DOI] [PubMed] [Google Scholar]
- Youatt J., Tham S. H. An enzyme system of Mycobacterium tuberculosis that reacts specifically with isoniazid. I. Am Rev Respir Dis. 1969 Jul;100(1):25–30. doi: 10.1164/arrd.1969.100.1.25. [DOI] [PubMed] [Google Scholar]
- Youatt J., Tham S. H. An enzyme system of Mycobacterium tuberculosis that reacts specifically with isoniazid. II. Correlation of this reaction with the binding and metabolism of isoniazid. Am Rev Respir Dis. 1969 Jul;100(1):31–37. doi: 10.1164/arrd.1969.100.1.31. [DOI] [PubMed] [Google Scholar]



