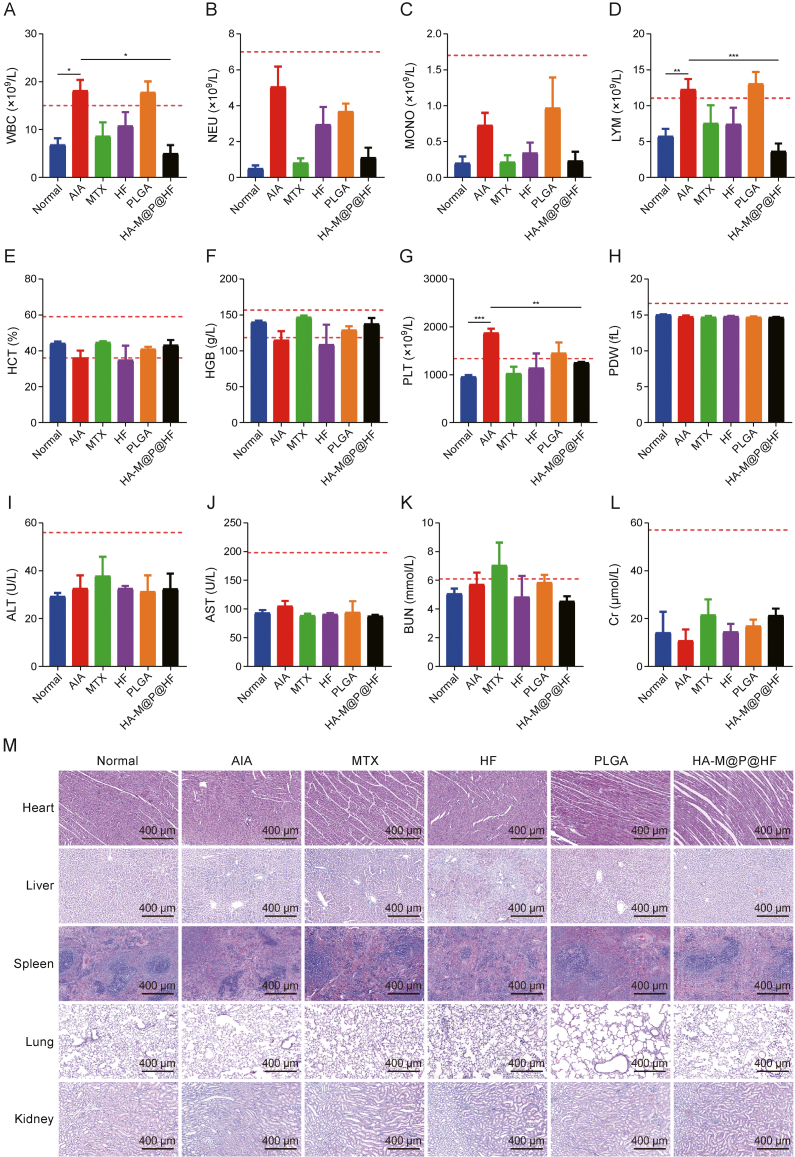
Fig. 9.
Safety evaluation of hyaluronic acid (HA)-modified hybrid membrane (M)-camouflaged poly lactic-co-glycolic acid (PLGA) loaded halofuginone hydrobromide (HF) nanoparticles (NPs) (HA-M@P@HF NPs) in vivo. (A–H) Complete blood panel analysis of white blood cell (WBC) (A), neutrophil (NEU) (B), monocyte (MONO) (C), lymphocyte (LYM) (D), hematocrit (HCT) (E), hemoglobin (HGB) (F), platelet (PLT) (G), and PLT distribution width (PDW) (H) levels. (I–L) Evaluation of hepatotoxicity and nephrotoxicity by measuring plasma levels of alanine transaminase (ALT) (I), aspartate aminotransferase (AST) (J), blood urea nitrogen (BUN) (K), and creatinine (Cr) (L). (M) Hematoxylin-eosin (H&E)-stained heart, liver, spleen, lung, and kidney tissue sections from normal control rats and adjuvant-induced arthritis (AIA) rats of different treatment groups. Data are presented as mean ± standard deviation (n = 3). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. MTX: methotrexate.