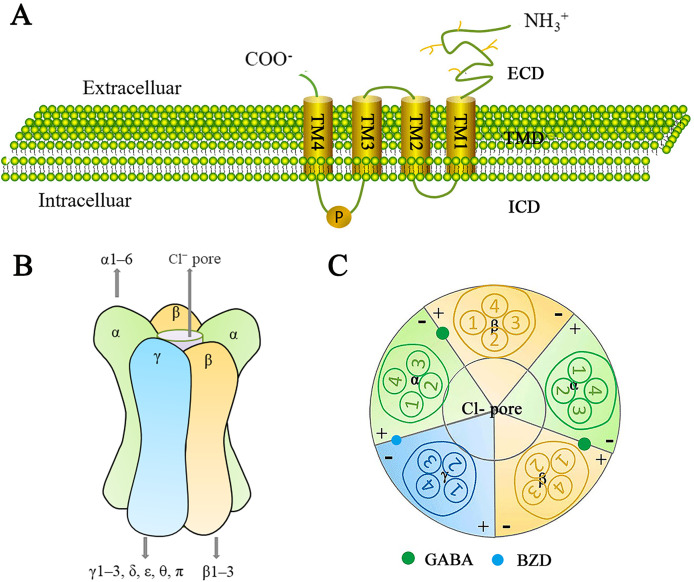
Figure 5. Schematic representation of the GABAA receptor and its subunits.
(A) The generic topological structure of a GABAA receptor subunit. The mature GABAA receptor comprises 450 amino acid residues. It possesses a large hydrophilic extracellular domain (ECD) at the N-terminus, four hydrophobic transmembrane domains (TMDs) (including TM1-TM4) in the middle, and a smaller intracellular domain (ICD) at the C-terminus. TM1 is connected to TM2 by a short intracellular loop; TM2 is connected to TM3 by a short extracellular loop; and TM3 is connected to TM4 by a long intracellular loop, which is phosphorylatable. (B) GABAA receptors are heteropentameric chloride-ion channels. They consist of five subunits, typically arranged in a heterodimeric fashion, forming a cylindrical channel. At the center of this channel is a pore permeable to chloride ions; the GABAA receptor exerts an inhibitory effect in the central nervous system by modulating the cell membrane’s depolarization. GABAA receptors are formed from 16 subunits: α1–6, β1–3, γ1–3, δ, ε, θ, and π. Functional GABA receptors contain at least one α-subunit, one β-subunit, and one γ-subunit. The most common pentameric combinations are 2α2β1γ, 2α1β2γ, and 1α2β2γ. 2α2β1γ is most frequently expressed in the central nervous system. (C) Distribution of GABA and benzodiazepine (BZD) binding sites in the GABAA receptor. Two GABA binding sites are located at the β+/α− interface, while one BZD binding site is located at the α+/γ− interface. Additionally, more than 10 potential ligand-binding sites are distributed throughout the receptor.