Abstract
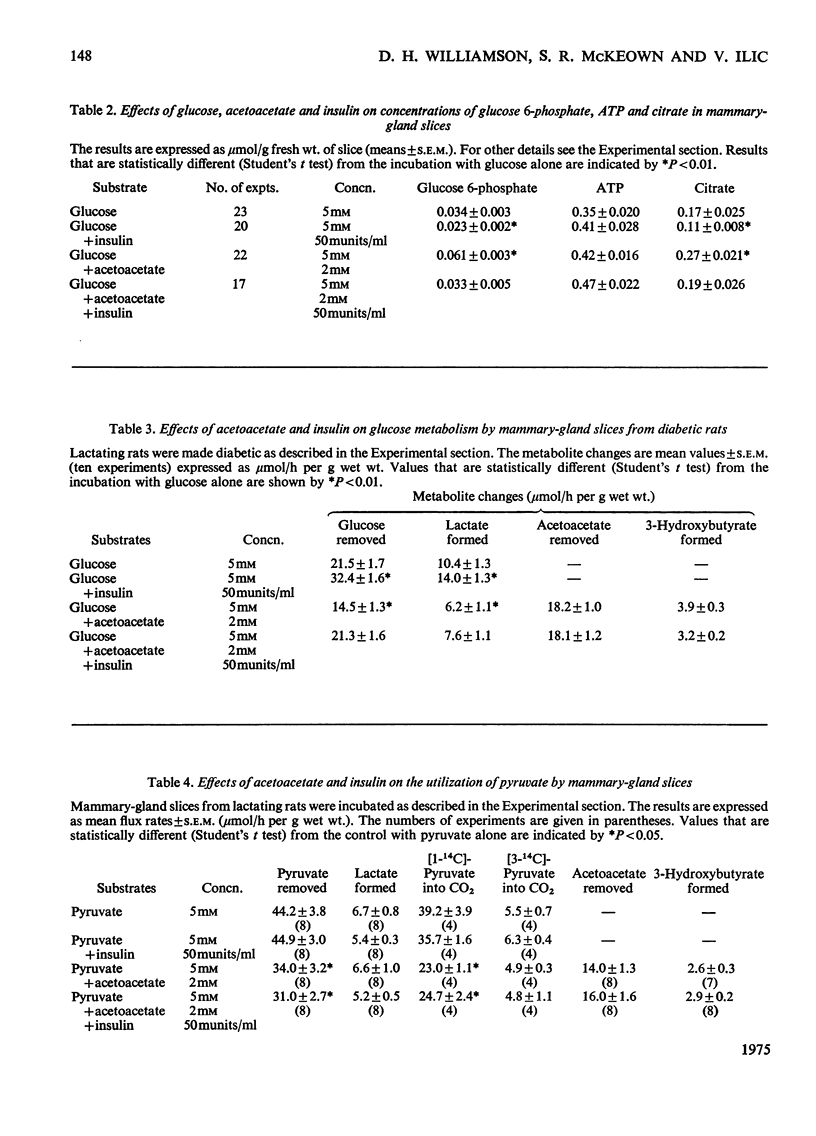
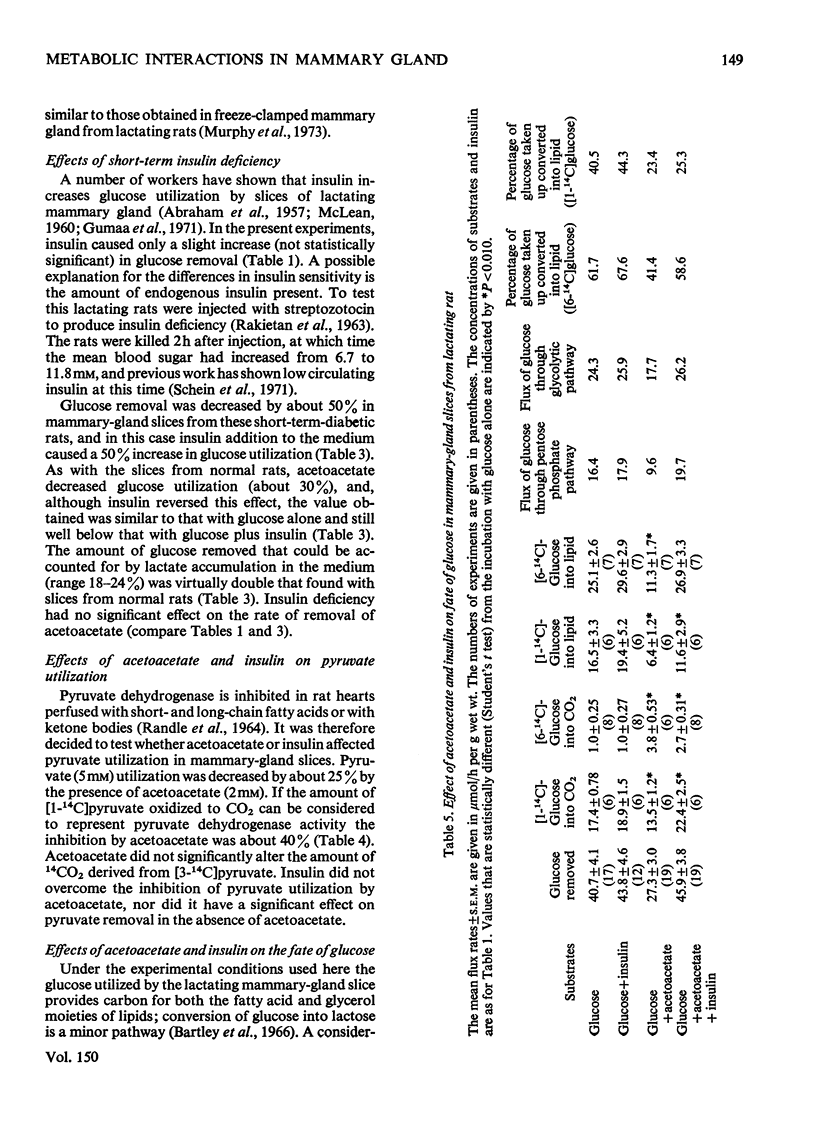
1. Utilization of 5mM-glucose by slices of lactating mammary gland was decreased 33% on addition of acetoacetate (2mM) to the incubation medium. This inhibition was accompanied by increases in the intracellular concentrations of citrate and glucose 6-phosphate. 2. In the presence of acetoacetates the accumulation of pyruvate in the medium approximately doubled. 3. Insulin completely reversed the inhibitory effect of acetoacetates on glucose utilization, without altering the amount of acetoacetate removed or pyruvate formed. 4. Similar results were obtained with mammary-gland slices from diabetic rats, except that insulin did not completely reverse the effects of acetoacetates. 5. Acetoacetate inhibited the formation of 14CO2 from [1-14C]pyruvate; this effect was not overcome by insulin. 6. Insulin increased the proportion of [3-14C]acetoacetate that was converted into lipid and decreased that oxidized to CO2.7. The physiological significance of these findings is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., CADY P., CHAIKOFF I. L. Effect of insulin in vitro in pathways of glucose utilization, other than Embden-Meyerhof, in rat mammary gland. J Biol Chem. 1957 Feb;224(2):955–962. [PubMed] [Google Scholar]
- Bartley J. C., Abraham S., Chaikoff I. L. Biosynthesis of lactose by mammary gland slices from the lactating rat. J Biol Chem. 1966 Mar 10;241(5):1132–1137. [PubMed] [Google Scholar]
- Carlson C. A., Kim K. H. Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. Arch Biochem Biophys. 1974 Oct;164(2):478–489. doi: 10.1016/0003-9861(74)90058-7. [DOI] [PubMed] [Google Scholar]
- Eggleston L. V., Krebs H. A. Regulation of the pentose phosphate cycle. Biochem J. 1974 Mar;138(3):425–435. doi: 10.1042/bj1380425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin A. R., Kuhn N. J. Aerobic lactate production by mammary tissue. Biochem J. 1975 Jan;146(1):273–275. doi: 10.1042/bj1460273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARLAND P. B., RANDLE P. J., NEWSHOLME E. A. CITRATE AS AN INTERMEDIARY IN THE INHIBITION OF PHOSPHOFRUCTOKINASE IN RAT HEART MUSCLE BY FATTY ACIDS, KETONE BODIES, PYRUVATE, DIABETES, AND STARVATION. Nature. 1963 Oct 12;200:169–170. doi: 10.1038/200169a0. [DOI] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum A. L., Darby F. J. The effect of adrenalectomy on the metabolism of the mammary glands of lactating rats. Biochem J. 1964 May;91(2):307–317. doi: 10.1042/bj0910307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Halestrap A. P., Denton R. M. Insulin and the regulation of adipose tissue acetyl-coenzyme A carboxylase. Biochem J. 1973 Mar;132(3):509–517. doi: 10.1042/bj1320509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins R. A., Williamson D. H. Measurements of substrate uptake by mammary gland of the rat. Biochem J. 1972 Oct;129(5):1171–1173. doi: 10.1042/bj1291171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Landau B. R., Bartsch G. E. The pentose cycle, triose phosphate isomerization, and lipogenesis in rat adipose tissue. J Biol Chem. 1966 Feb 10;241(3):727–740. [PubMed] [Google Scholar]
- Katz J., Wals P. A. Pentose cycle and reducing equivalents in rat mammary-gland slices. Biochem J. 1972 Jul;128(4):879–899. doi: 10.1042/bj1280879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Wals P. A., Van de Velde R. L. Lipogenesis by acini from mammary gland of lactating rats. J Biol Chem. 1974 Nov 25;249(22):7348–7357. [PubMed] [Google Scholar]
- Kinsella J. E. Biosynthesis of lipids from (2-14C)acetate and D(-)-beta-hydroxy(1,3-14C)butyrate by mammary cells from bovine and rat. Biochim Biophys Acta. 1970 Jun 9;210(1):28–38. doi: 10.1016/0005-2760(70)90058-5. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Eggleston L. V. Metabolism of acetoacetate in animal tissues. 1. Biochem J. 1945;39(5):408–419. [PMC free article] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Weidemann M. J., Speake R. N. The fate of isotopic carbon in kidney cortex synthesizing glucose from lactate. Biochem J. 1966 Oct;101(1):242–249. doi: 10.1042/bj1010242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakou S. Y., Kuhn N. J. Lactogenesis in the diabetic rat. J Endocrinol. 1973 Oct;59(1):199–200. doi: 10.1677/joe.0.0590199. [DOI] [PubMed] [Google Scholar]
- Martin R. J., Baldwin R. L. Effects of alloxan diabetes on lactational performance and mammary tissue metabolism in the rat. Endocrinology. 1971 Apr;88(4):863–867. doi: 10.1210/endo-88-4-863. [DOI] [PubMed] [Google Scholar]
- McLean P., Greenbaum A. L., Gumaa K. A. Constant and specific proportion groups of enzymes in rat mammary gland and adipose tissue in relation to lipogenesis. FEBS Lett. 1972 Feb 15;20(3):277–282. doi: 10.1016/0014-5793(72)80086-3. [DOI] [PubMed] [Google Scholar]
- Murphy G., Ariyanayagam A. D., Kuhn N. J. Progesterone and the metabolic control of the lactose biosynthetic pathway during lactogenesis in the rat. Biochem J. 1973 Dec;136(4):1105–1116. doi: 10.1042/bj1361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Randle P. J. Regulation of glucose uptake by muscle. 7. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes, starvation, hypophysectomy and adrenalectomy, on the concentrations of hexose phosphates, nucleotides and inorganic phosphate in perfused rat heart. Biochem J. 1964 Dec;93(3):641–651. doi: 10.1042/bj0930641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARMEGGIANI A., BOWMAN R. H. REGULATION OF PHOSPHOFRUCTOKINASE ACTIVITY BY CITRATE IN NORMAL AND DIABETIC MUSCLE. Biochem Biophys Res Commun. 1963 Aug 1;12:268–273. doi: 10.1016/0006-291x(63)90294-8. [DOI] [PubMed] [Google Scholar]
- Page M. A., Williamson D. H. Lactating mammary gland of the rat: a potential major site of ketone-body utilization. Biochem J. 1972 Jun;128(2):459–460. doi: 10.1042/bj1280459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAKIETEN N., RAKIETEN M. L., NADKARNI M. V. Studies on the diabetogenic action of streptozotocin (NSC-37917). Cancer Chemother Rep. 1963 May;29:91–98. [PubMed] [Google Scholar]
- Randle P. J., Newsholme E. A., Garland P. B. Regulation of glucose uptake by muscle. 8. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles. Biochem J. 1964 Dec;93(3):652–665. doi: 10.1042/bj0930652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapag-Hagar M., Lagunas R., Sols A. Apparent unbalance between the activities of 6-phosphogluconate and glucose-6-phosphate dehydrogenases in rat liver. Biochem Biophys Res Commun. 1973 Jan 4;50(1):179–185. doi: 10.1016/0006-291x(73)91080-2. [DOI] [PubMed] [Google Scholar]
- Schein P. S., Alberti K. G., Williamson D. H. Effects of streptozotocin on carbohydrate and lipid metabolism in the rat. Endocrinology. 1971 Sep;89(3):827–834. doi: 10.1210/endo-89-3-827. [DOI] [PubMed] [Google Scholar]
- Thompson M. P., Williamson D. H. Metabolic interactions of glucose, acetoacetate and adrenaline in rat submaxillary gland in vitro. Biochem J. 1975 Mar;146(3):635–644. doi: 10.1042/bj1460635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON J. R., KREBS H. A. Acetoacetate as fuel of respiration in the perfused rat heart. Biochem J. 1961 Sep;80:540–547. doi: 10.1042/bj0800540. [DOI] [PMC free article] [PubMed] [Google Scholar]


