Abstract
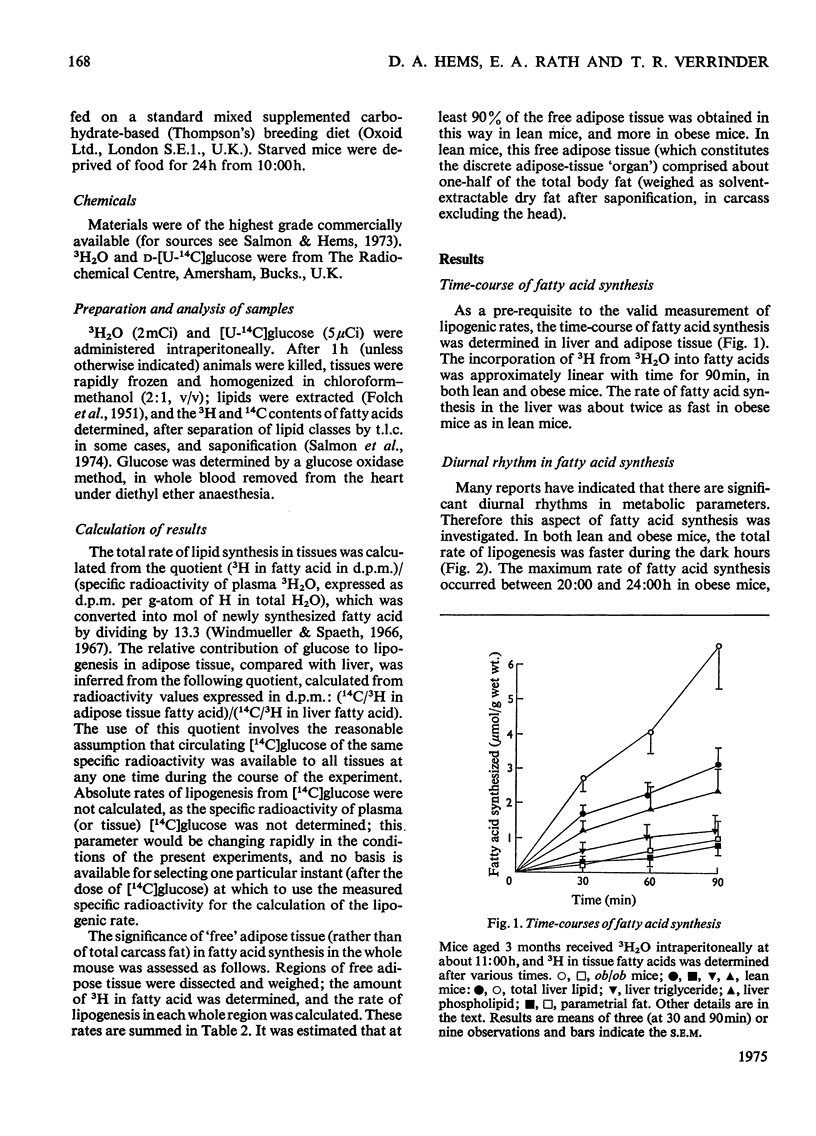
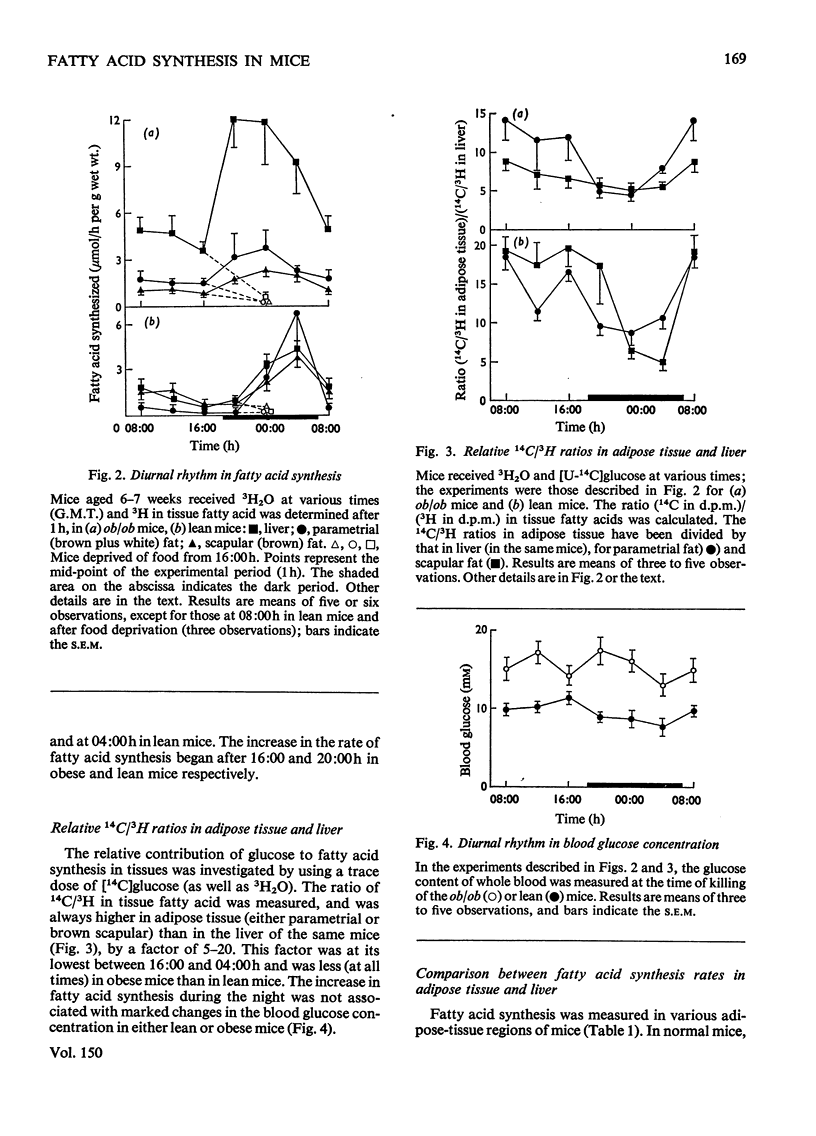
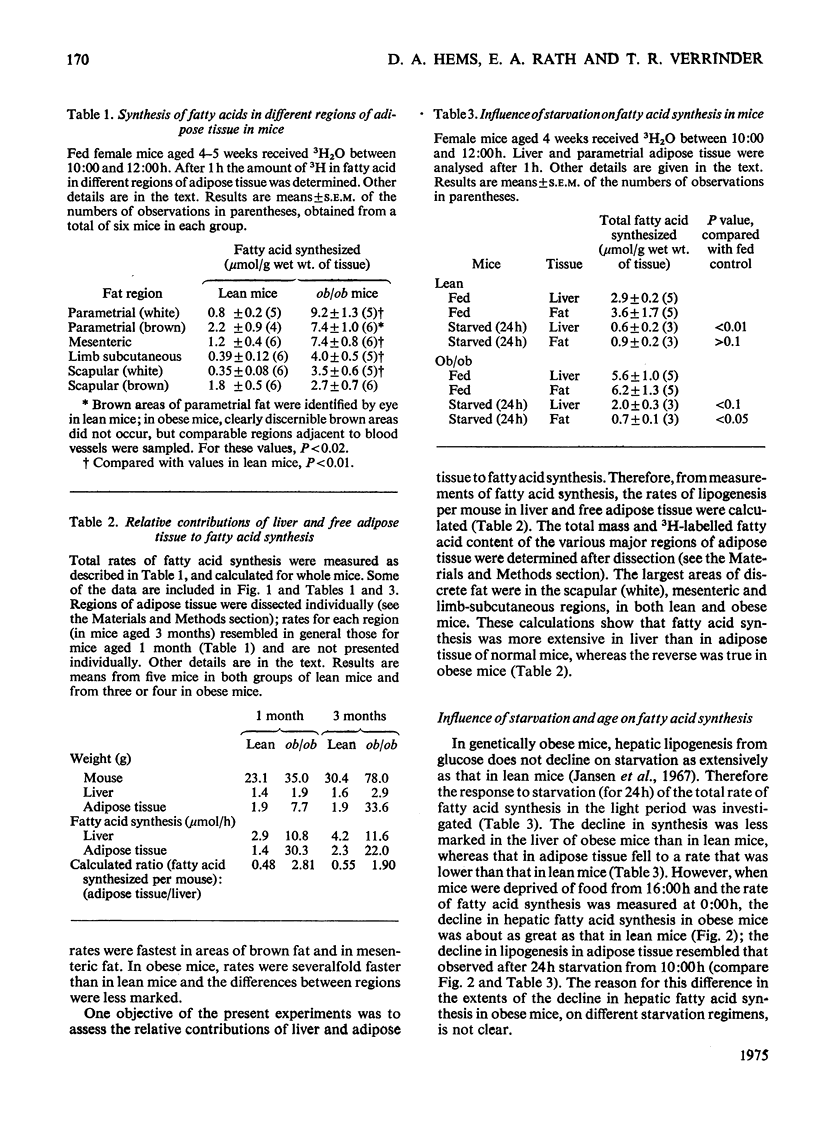
1. The synthesis of long-chain fatty acids de novo was measured in the liver and in regions of adipose tissue in intact normal and genetically obses mice throughout the daily 24h cycle. 2. The total rate of synthesis, as measured by the rate of incorporation of 3H from 3H2O into fatty acid, was highest during the dark period, in liver and adipose tissue of lean or obese mice. 3. The rate of incorporation of 14C from [U-14C]glucose into fatty acid was also followed (in the same mice). The 14C/3H ratios were higher by a factor of 5-20 in parametrial and scapular fat than that in liver. This difference was less marked during the dark period (of maximum fatty acid synthesis). 4. In normal mice, the total rate of fatty acid synthesis in the liver was about twofold greater than that in all adipose tissue regions combined. 5. In obese mice, the rate of fatty acid synthesis was more rapid than in lean mice, in both liver and adipose tissue. Most of the extra lipogenesis occurred in adipose tissue. The extra hepatic fatty acids synthesized in obese mice were located in triglyceride rather than phospholipid. 6. In adipose tissue of normal mice, the rate of fatty acid synthesis was most rapid in the intra-abdominal areas and in brown fat. In obese mice, all regions exhibited rapid rates of fatty acid synthesis. 7. These results shed light on the relative significance of liver and adipose tissue (i.e. the adipose 'organ') in fatty acid synthesis in mice, on the mino importance of glucose in hepatic lipogenesis, and on the alterations in the rate of fatty acid synthesis in genetically obese mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R. R., Beloff-Chain A. Hormonal control of intermediary metabolism in obese hyperglycemic mice. I. The sensitivity and response to insulin in adipose tissue and muscle in vitro. Diabetes. 1971 Aug;20(8):522–534. doi: 10.2337/diab.20.8.522. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., Singh A., Le Marchand Y., Loten E. G., Jeanrenaud B. Abnormalities in lipogenesis and triglyceride secretion by perfused livers of obese-hyperglycaemic (ob-ob) mice: relationship with hyperinsulinaemia. Diabetologia. 1974 Apr;10(2):155–162. doi: 10.1007/BF01219673. [DOI] [PubMed] [Google Scholar]
- Baker N., Huebotter R. J. Lipogenic activation after nibbling and gorging in mice. J Lipid Res. 1973 Jan;14(1):87–94. [PubMed] [Google Scholar]
- Baker N., Huebotter R. J. Rapid activation and inactivation of fatty acid synthesis from glucose in vivo. J Lipid Res. 1972 May;13(3):329–337. [PubMed] [Google Scholar]
- Baker N., Huebotter R. J. Specific role of glucose in rapid lipogenic activation in vivo. J Lipid Res. 1973 Jan;14(1):95–101. [PubMed] [Google Scholar]
- Bruckdorfer K. R., Kang S. S., Khan I. H., Bourne A. R., Yudkin J. Diurnal changes in the concentrations of plasma lipids, sugars, insulin and corticosterone in rats fed diets containing various carbohydrates. Horm Metab Res. 1974 Mar;6(2):99–106. doi: 10.1055/s-0028-1093890. [DOI] [PubMed] [Google Scholar]
- Brunengraber H., Boutry M., Lowenstein J. M. Fatty acid and 3- -hydroxysterol synthesis in the perfused rat liver. Including measurements on the production of lactate, pyruvate, -hydroxy-butyrate, and acetoacetate by the fed liver. J Biol Chem. 1973 Apr 25;248(8):2656–2669. [PubMed] [Google Scholar]
- Christophe J., Furnelle J., Boutry M., Winand J. Qualite des lipides et quantite des proteines synthetises in vivo par la souris normale et la souris obbése-hyperglycemique de Bar Harbor. Bull Soc Chim Biol (Paris) 1970 Apr 17;52(3):333–348. [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Lipogenesis in rat hepatocytes. J Biol Chem. 1974 Apr 10;249(7):2028–2036. [PubMed] [Google Scholar]
- Edwards P. A., Muroya H., Gould R. G. In vivo demonstration of the circadian thythm of cholesterol biosynthesis in the liver and intestine of the rat. J Lipid Res. 1972 May;13(3):396–401. [PubMed] [Google Scholar]
- Elliott J., Dade E., Salmon D. M., Hems D. A. Hepatic metabolism in normal and genetically obese mice. Biochim Biophys Acta. 1974 Apr 22;343(2):307–323. doi: 10.1016/0304-4165(74)90095-6. [DOI] [PubMed] [Google Scholar]
- Elliott J., Hems D. A., Beloff-Chain A. Carbohydrate metabolism of the isolated perfused liver of normal and genetically obese--hyperglycaemic (ob-ob) mice. Biochem J. 1971 Dec;125(3):773–780. doi: 10.1042/bj1250773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Haft D. E. Role of plasma triglyceride turnover in deposition of newly synthesized fatty acid in adipose tissue. Horm Metab Res. 1973 Nov;5(6):449–453. doi: 10.1055/s-0028-1093905. [DOI] [PubMed] [Google Scholar]
- Jansen G. R., Zanetti M. E., Hutchison C. F. Studies in lipogenesis in vivo: Fatty acid and cholesterol synthesis during starvation and re-feeding. Biochem J. 1966 Dec;101(3):811–818. doi: 10.1042/bj1010811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen G. R., Zanetti M. E., Hutchison C. F. Studies on lipogenesis in vivo: Fatty acid and cholesterol synthesis in hyperglycaemic-obese mice. Biochem J. 1967 Mar;102(3):870–877. doi: 10.1042/bj1020870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungas R. L. Fatty acid synthesis in adipose tissue incubated in tritiated water. Biochemistry. 1968 Oct;7(10):3708–3717. doi: 10.1021/bi00850a050. [DOI] [PubMed] [Google Scholar]
- Katz J., Wals P. A. Lipogenesis from lactate in rat adipose tissue. Biochim Biophys Acta. 1974 Jun 26;348(3):344–356. doi: 10.1016/0005-2760(74)90214-8. [DOI] [PubMed] [Google Scholar]
- Kimura T., Maji T., Ashida K. Periodicity of food intake and lipogenesis in rats subjected to two different feeding plans. J Nutr. 1970 Jun;100(6):691–697. doi: 10.1093/jn/100.6.691. [DOI] [PubMed] [Google Scholar]
- Loten E. G., Rabinovitch A., Jeanrenaud B. In vivo studies on lipogenesis in obese hyperglycaemic (ob-ob) mice: possible role of hyperinsulinaemia. Diabetologia. 1974 Feb;10(1):45–52. doi: 10.1007/BF00421413. [DOI] [PubMed] [Google Scholar]
- Lowenstein J. M. Effect of (-)-hydroxycitrate on fatty acid synthesis by rat liver in vivo. J Biol Chem. 1971 Feb 10;246(3):629–632. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem. 1971 Feb 25;246(4):1149–1159. [PubMed] [Google Scholar]
- Nowell N. W. Circadian rhythm of glucose tolerance in laboratory mice. Diabetologia. 1970 Oct;6(5):488–492. doi: 10.1007/BF01211889. [DOI] [PubMed] [Google Scholar]
- Salmon D. M., Bowen N. L., Hems D. A. Synthesis of fatty acids in the perused mouse liver. Biochem J. 1974 Sep;142(3):611–618. doi: 10.1042/bj1420611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D. M., Hems D. A. Plasma lipoproteins and the synthesis and turnover of plasma triglyceride in normal and genetically obese mice. Biochem J. 1973 Nov;136(3):551–563. doi: 10.1042/bj1360551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shreeve W. W., Lamdin E., Oji N., Slavinski R. Biosynthesis of fatty acids in obese mice in vivo. I. Studies with glucose-1-3-H(1-14-C), glucose-6-3-H(6-14-C), DL-lactate-2-3-H(2-14-C), and glycerol-2-3-H(1,3-14-C). Biochemistry. 1967 Apr;6(4):1160–1167. doi: 10.1021/bi00856a028. [DOI] [PubMed] [Google Scholar]
- Winand J., Furnelle J., Christophe J. Le métabolisme lipidique du foie chez la souris normale et la souris obèse-hyperglycémique. Biochim Biophys Acta. 1968 Mar 4;152(2):280–292. [PubMed] [Google Scholar]
- Winand J., Furnelle J., Wodon C., Hebbelinck M., Christophe J. 7-Day time study of lipid metabolism in normal and obese-hyperglycemic Bar Harbor mice. Qualitative and quantitative aspects. Biochimie. 1973;55(1):63–73. doi: 10.1016/s0300-9084(73)80238-x. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. De novo synthesis of fatty acid in perfused rat liver as a determinant of plasma lipoprotein production. Arch Biochem Biophys. 1967 Nov;122(2):362–369. doi: 10.1016/0003-9861(67)90206-8. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Perfusion in situ with tritium oxide to measure hepatic lipogenesis and lipid secretion. Normal and orotic acid-fed rats. J Biol Chem. 1966 Jun 25;241(12):2891–2899. [PubMed] [Google Scholar]
- Yen T. T., Steinmetz J. A. Lipolysis of genetically obese and-or hyperglycemic mice with reference to insulin response of adipose tissue. Horm Metab Res. 1972 Sep;4(5):331–337. doi: 10.1055/s-0028-1094027. [DOI] [PubMed] [Google Scholar]


