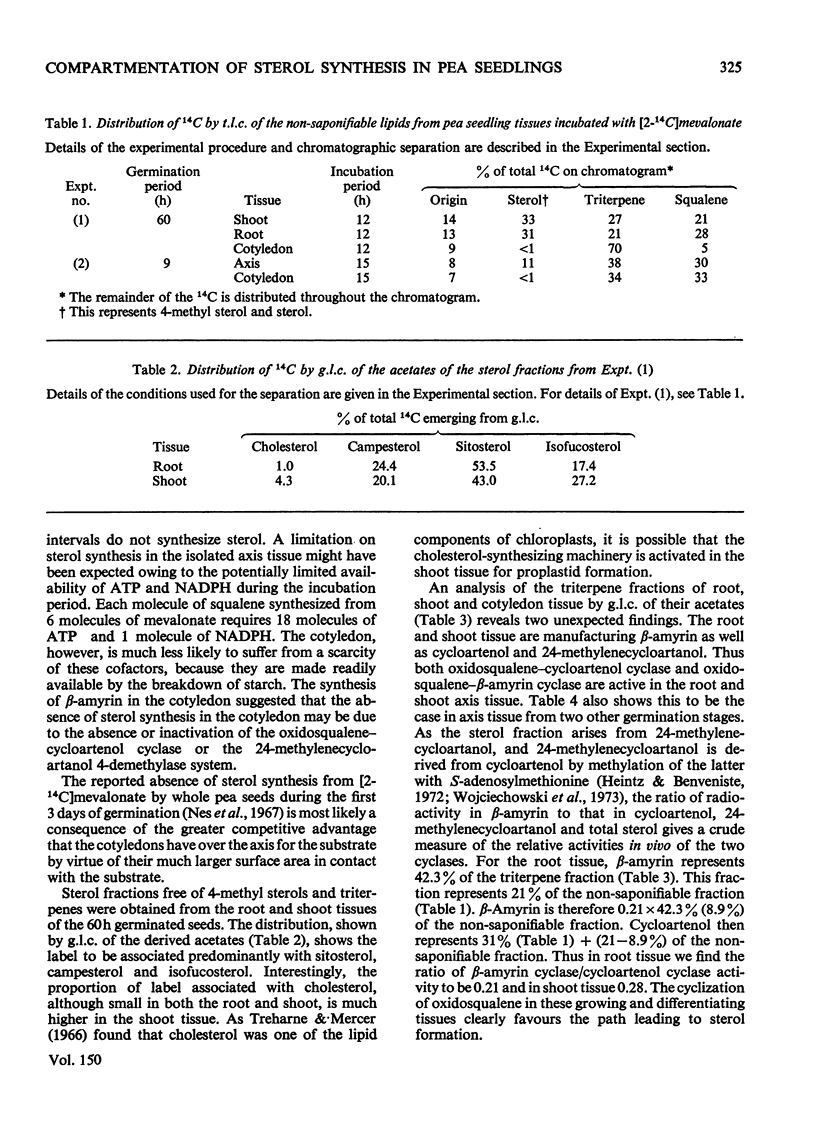
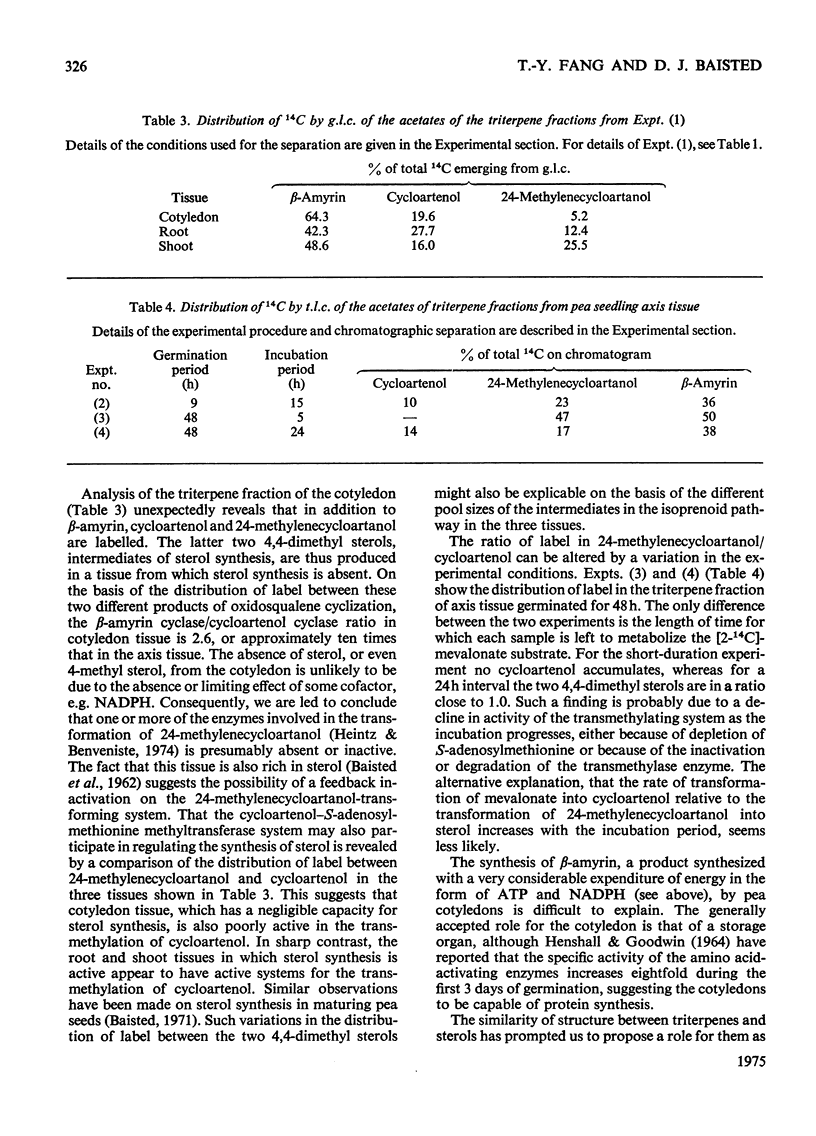
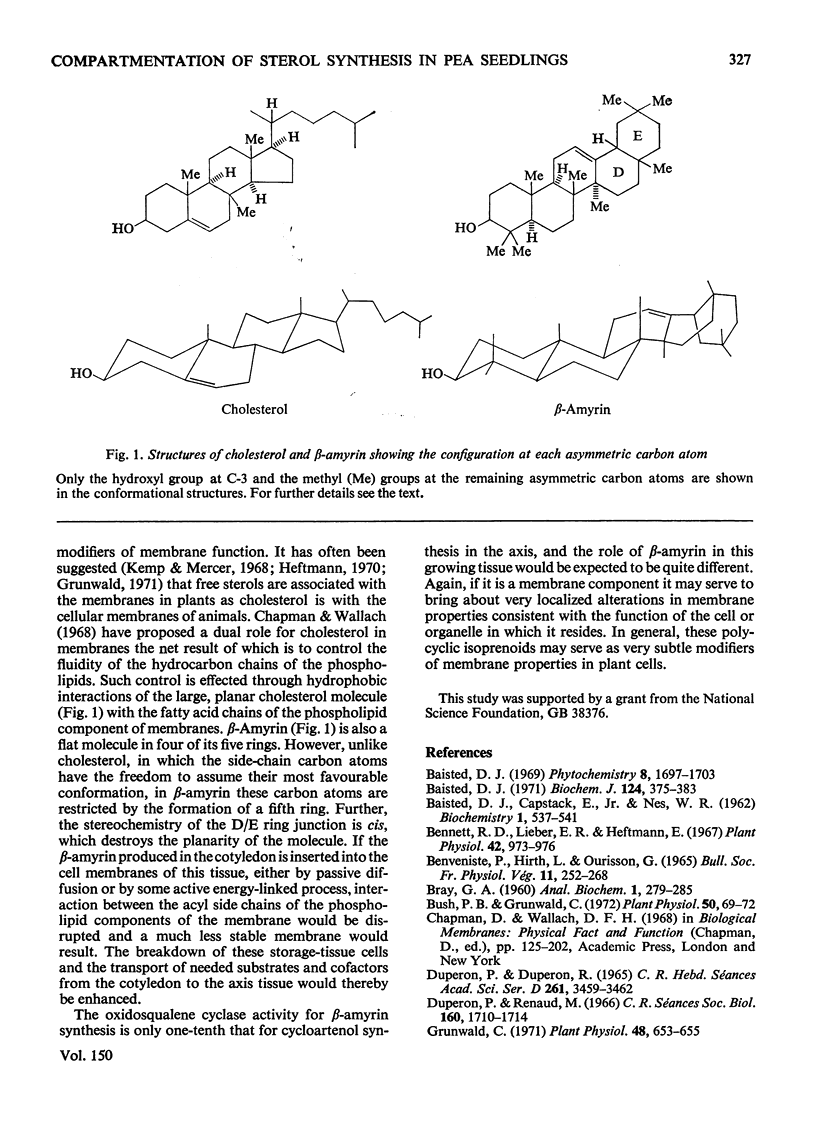
Abstract
Axis tissues, root and shoot, of germinating pea seedlings actively synthesize sterol from [2-14C]mevalonate during the first 3 days of germination. In addition to the intermediates of sterol synthesis, cycloartenol and 24-methylenecycloartanol, these tissues also form the triterpene beta-amyrin. The cyclase catalysing the formation of cycloartenol from oxidosqualene is about four times as active as that for beta-amyrin synthesis. 2. Sterol synthesis in the cotyledon is negligible, but cycloartenol and 24-methylenecycloartanol, as well as beta-amyrin, are synthesized there. Oxidosqualene cyclase activity in this tissue is 2.6 times as active for beta-amyrin synthesis as for cycloartenol synthesis. 3. Comparison of the relative amounts of 14C in cycloartenol and 24-methylenecycloartanol in the axis tissues and cotyledons of 3-day-old seedlings point to relatively active cycloartenol-S-adenosylmethionine methyltransferase systems in both axis tissues and a poorly active system in the cotyledon. 4. The role of beta-amyrin synthesis in the germinating pea seedling is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAISTED D. J., CAPSTACK E., Jr, NES W. R. The biosynthesis of beta-amyrin and beta-sitosterol in germinating seeds of Pisum sativum. Biochemistry. 1962 May 25;1:537–541. doi: 10.1021/bi00909a027. [DOI] [PubMed] [Google Scholar]
- Baisted D. J. Sterol and triterpene synthesis in the developing and germinating pea seed. Biochem J. 1971 Sep;124(2):375–383. doi: 10.1042/bj1240375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett R. D., Lieber E. R., Heftmann E. Time Course of Steroid Biosynthesis and Metabolism in Haplopappus heterophyllus. Plant Physiol. 1967 Jul;42(7):973–976. doi: 10.1104/pp.42.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush P. B., Grunwald C. Sterol Changes during Germination of Nicotiana tabacum Seeds. Plant Physiol. 1972 Jul;50(1):69–72. doi: 10.1104/pp.50.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunwald C. Effects of free sterols, steryl ester, and steryl glycoside on membrane permeability. Plant Physiol. 1971 Nov;48(5):653–655. doi: 10.1104/pp.48.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz R., Benveniste P. Plant sterol metabolism. Enzymatic cleavage of the 9beta, 19beta-cyclopropane ring of cyclopropyl sterols in bramble tissue cultures. J Biol Chem. 1974 Jul 10;249(13):4267–4274. [PubMed] [Google Scholar]
- Kasprzyk Z., Sliwowski J., Boleslawska-Kokosza D. The variations of triterpenoids in germinating seeds of Calendula officinalis. Acta Biochim Pol. 1970;17(1):11–18. [PubMed] [Google Scholar]
- Kemp R. J., Mercer E. I. Studies on the sterols and sterol esters of the intracellular organelles of maize shoots. Biochem J. 1968 Nov;110(1):119–125. doi: 10.1042/bj1100119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciechowski Z. A., Goad L. J., Goodwin T. W. S-adenosyl-L-methionine-cycloartenol methyltransferase activity in cell-free systems from Trebouxia sp. and Scenedesmus obliquus. Biochem J. 1973 Oct;136(2):405–412. doi: 10.1042/bj1360405. [DOI] [PMC free article] [PubMed] [Google Scholar]


