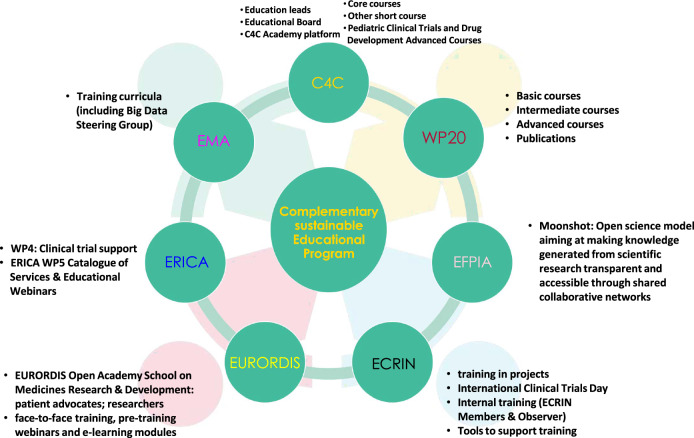
Fig. 1.
An overview of the educational programs for rare disease clinical trials methodologies. Key components include various institutions active participation such as the European Medicines Agency (EMA), connect 4 children (C4C), Work Package 20 (WP20) of the European Joint Programme on Rare Diseases, European Federation of Pharmaceutical Industries and Associations (EFPIA), European Clinical Research Infrastructure Network (ECRIN), European Organisation for RD (EURORDIS), and European Rare Disease Research Coordination and Support Action (ERICA). Educational programs in RD Clinical Trial methodologies. This figure provides an overview of the various components within the Complementary Sustainable Educational Program under the WP20 initiative. It highlights key educational offerings such as training curricula, the C4C Academy platform, and various levels of courses (basic, intermediate, advanced). The figure also outlines the contributions of different entities including connect 4 children (C4C), European Federation of Pharmaceutical Industries and Associations (EFPIA), European Clinical Research Infrastructure Network (ECRIN), European Organisation for RD (EURORDIS), and European Rare Disease Research Coordination and Support Action (ERICA). Additionally, it details specific programs like the Moonshot initiative, which aims to enhance the transparency and accessibility of scientific knowledge through collaborative networks. Other features include face-to-face training, pre-training webinars, e-learning modules, and a range of specialized courses tailored to support clinical trial training