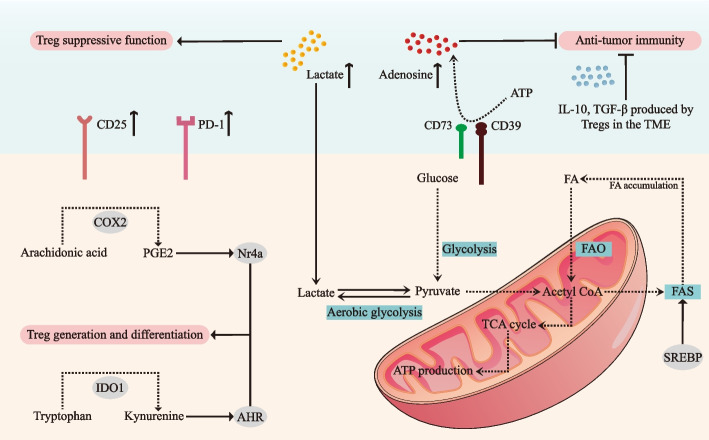
Fig. 2.
Metabolic and immunosuppressive roles of Treg cells in the tumor microenvironment of OSCC. The tumor microenvironment (TME) of oral squamous cell carcinoma (OSCC) is characterized by elevated levels of immunosuppressive metabolites, with lactate accumulation from enhanced aerobic glycolysis and increased adenosine generation through CD39/CD73-mediated ATP hydrolysis, suppress the anti-tumor immune functions. To maintain their suppressive capabilities, Tregs undergo significant metabolic reprogramming, characterized by enhanced fatty acid oxidation (FAO) and fatty acid synthesis (FAS) pathways, regulated by sterol regulatory element-binding proteins (SREBPs). The metabolic interplay is further complicated by competition between Tregs and tumor cells for essential nutrients, including glucose and fatty acids, creating a metabolically hostile environment for anti-tumor immune responses. Critical molecular pathways, including the indoleamine 2,3-dioxygenase 1 (IDO1)-mediated conversion of tryptophan to kynurenine, activate the aryl hydrocarbon receptor (AHR) to promote Treg differentiation, while cyclooxygenase-2 (COX-2)-derived prostaglandin E2 (PGE2) enhances Treg accumulation through nuclear receptor 4a (Nr4a) upregulation. Tregs also secrete immunosuppressive cytokines, including IL-10, TGF-β, and IL-35, which suppress effector immune responses and facilitate tumor progression. Further, Tregs employ PD-1 and CD25 to maintain their suppressive phenotype and survival under the metabolically hostile conditions of the TME. TME: tumor microenvironment; OSCC: oral squamous cell carcinoma; FAO: fatty acid oxidation; FAS: fatty acid synthesis; SREBP: sterol regulatory element-binding protein; IDO1: Indoleamine 2, 3-dioxygenase 1; AHR: aryl hydrocarbon receptor; COX-2: cyclooxygenase-2; PGE2: prostaglandin E2; Nr4a: nuclear receptor 4a