Fig. 1.
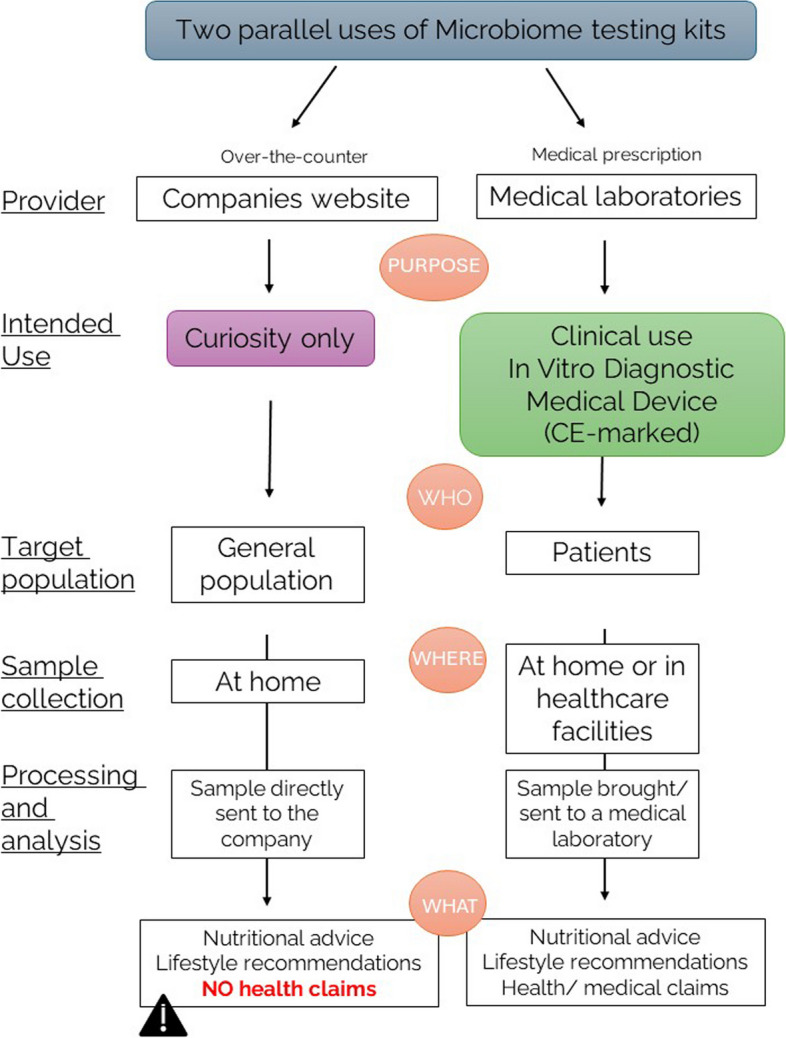
Two different regulatory statuses, depending on the intended use, need to be proposed for the microbiome testing placed on the European market. In terms of intended use, two different microbiome testing kits can be proposed, but currently, the distinction between these two different purposes is still unclear and undefined. In conclusion of a multistakeholder workshop, two different frameworks to separate the microbiome testing kits intended for consumer curiosity only and the microbiome testing kits intended for clinical use and diagnostic were proposed. Microbiome testing kits, designed to satisfy consumer curiosity, can be purchased over the counter (as is currently the case) and are being proposed to the general population. The sample to be analysed is collected at home and sent directly to the company offering the analysis. Based on the results and analysis, general recommendations (dietary or lifestyle recommendations) can be made directly to the consumer, without the assistance of a healthcare professional. However, no health claims or health recommendations can be made in the report. On the other hand, microbiome testing kits intended for clinical use should be regulated as in vitro diagnostic medical devices that comply with the currently applicable regulation on the medical devices (Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU). These devices are intended for a diagnostic purpose and should therefore require a medical prescription. The sample can be collected at home and brought directly or sent to a medical laboratory. In this context, medical claims can be included in the report, which should be sent to a doctor or health professional
