FIGURE 1.
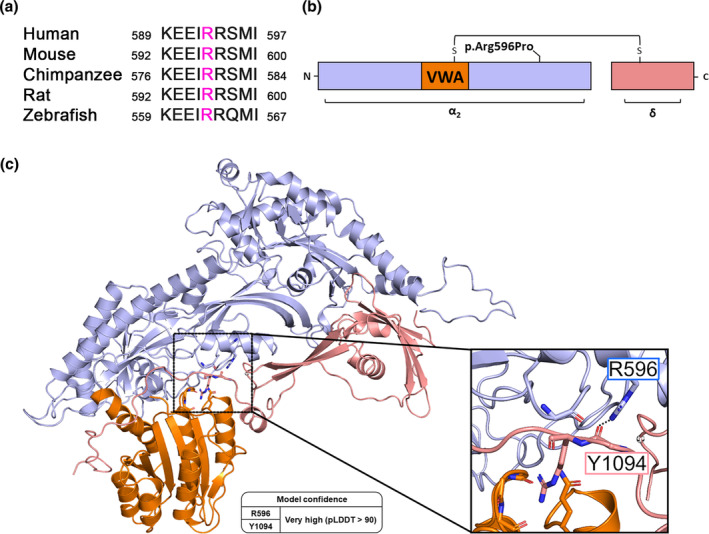
The conserved R596 residue is predicted to have a critical role in stabilizing the interaction between α2 and δ peptides. (a) Sequence alignment between α2δ‐2 proteins from different species. Position corresponding to human arginine 593 (R596 in mouse α2δ‐2) is highlighted in purple. (b) Schematic overview of α2δ‐2 protein illustrating the positions of the von Willebrand factor type A domain (VWA) and the p.R596P mutation. (c) Structural homology modeling of mouse α2δ‐2 protein based on the published Cryo‐EM structure of the human CaV2.2 channel complex (PDB code: 7MIY) predicts a critical interaction of the side chain of R596 (α2 peptide) with the backbone of Y1094 residue (δ peptide) through a hydrogen bond. (Color code: α2 peptide in purple, VWA domain in orange, and δ peptide in pink.) AlphaFold per‐residue model confidence scores (pLDDT) for R596 and Y1094 residues are very high (97.38 and 94.25, respectively).
