FIGURE 2.
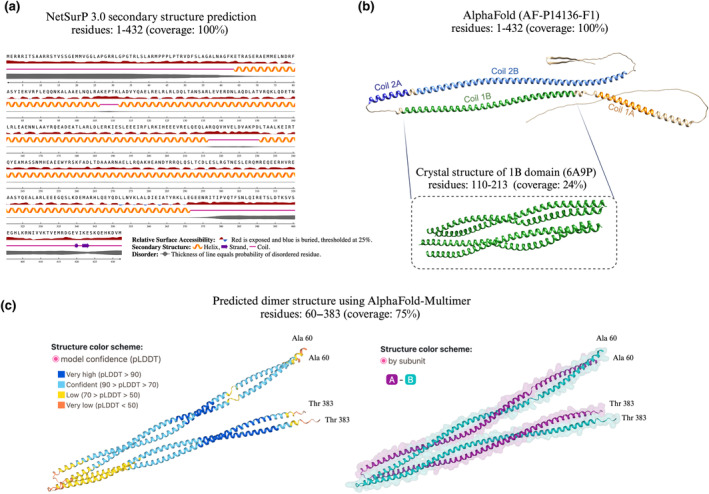
Structural characteristics of mono‐ and dimeric GFAP. Our understanding of the 3‐dimensional (3D) structure of GFAP is limited. (a) The predicted secondary structural components of GFAP using NetSurfP 3.0 covering 100% of the full‐length GFAP AA sequence (Høie et al., 2022; Klausen et al., 2019). (b) The 3D structure of GFAP predicted by AlphaFold covering 100% of the full‐length protein sequence composed of four coils: 1A, 1B, 2A, 2B (Jumper et al., 2021). Below the AlphaFold structure, the X‐ray PDB structure of the 1B domain of GFAP is displayed and determined to form a homotetramer covering 24% of the protein sequence (Kim et al., 2018). (c) The predicted dimer structure of recombinant GFAP using AlphaFold‐Multimer (Evans et al., 2021; Gogishvili et al., 2023) visualised with the PAE viewer tool (Elfmann & Stülke, 2023). Data obtained from hydrogen‐deuterium exchange measurements support the existence of this structure (Gogishvili et al., 2023).
