Abstract
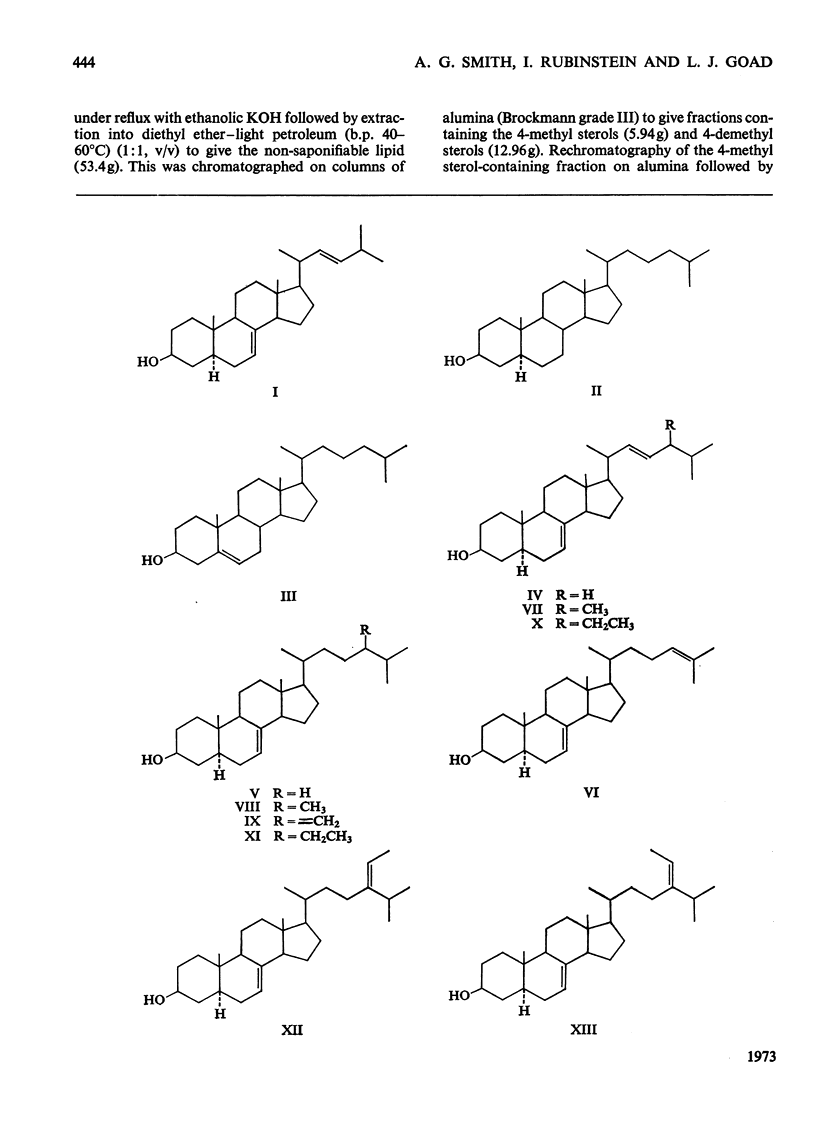
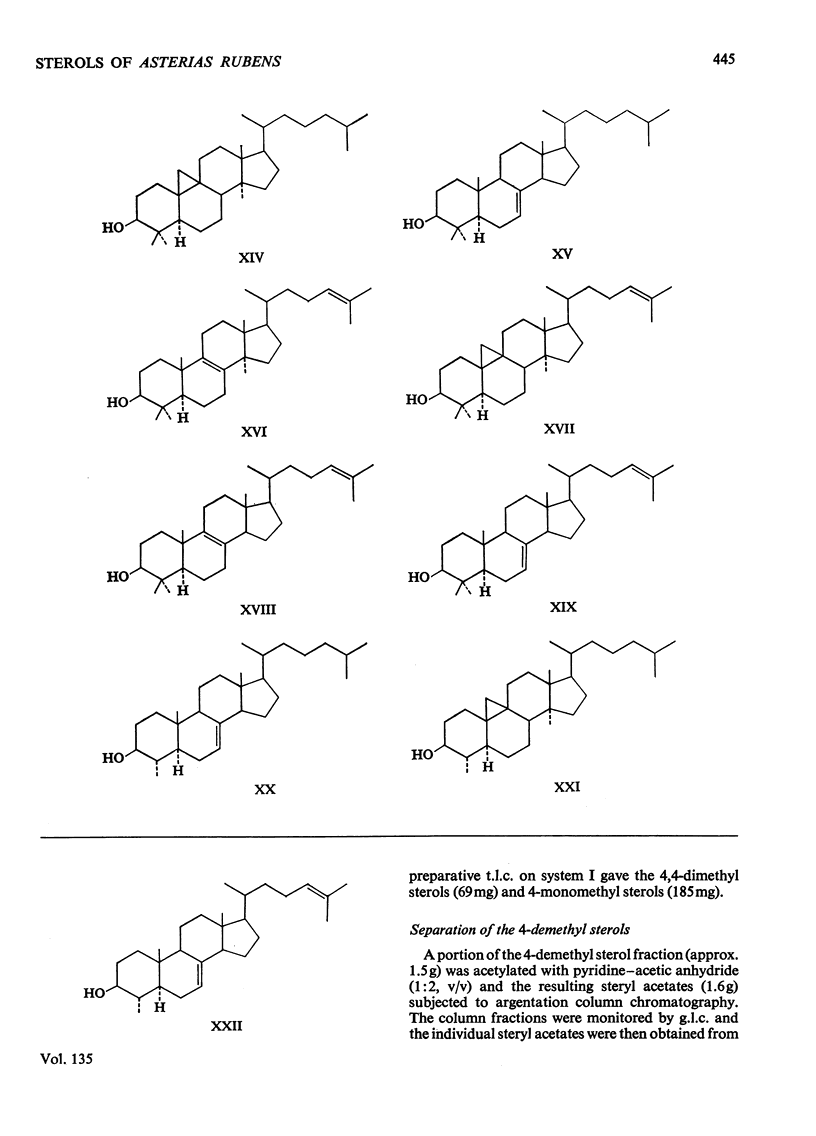
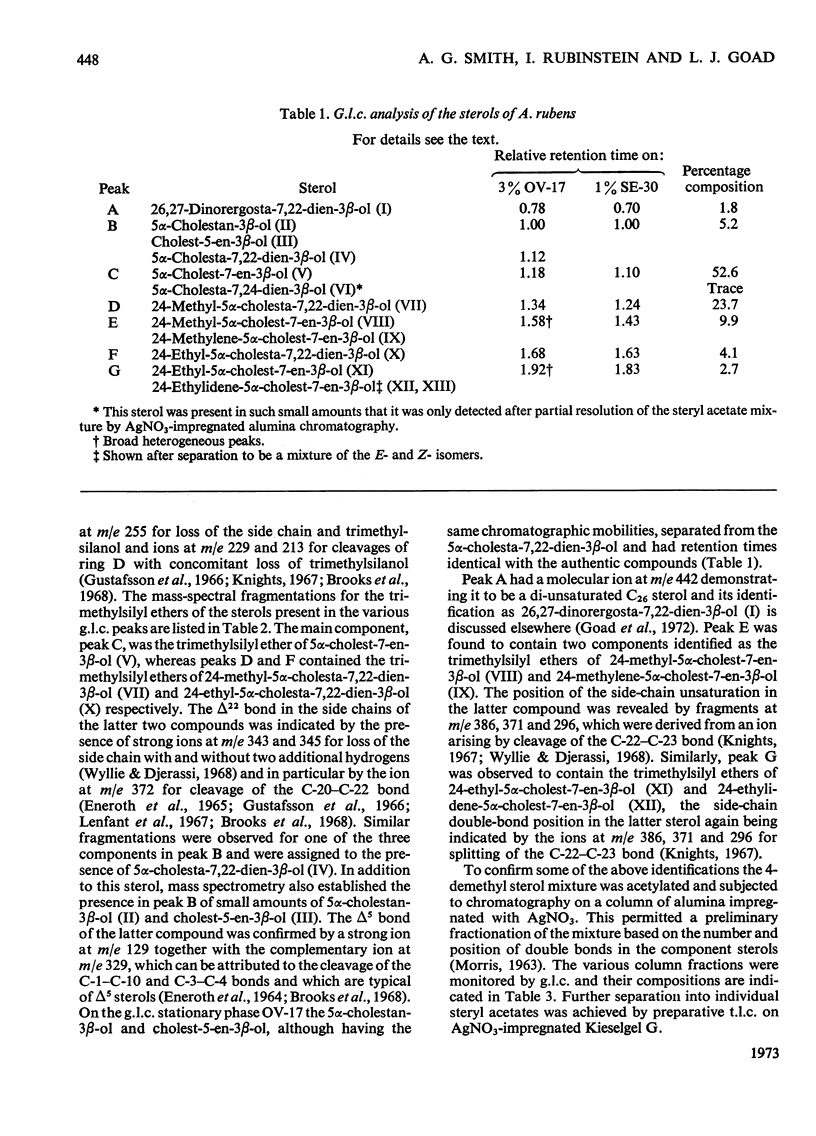
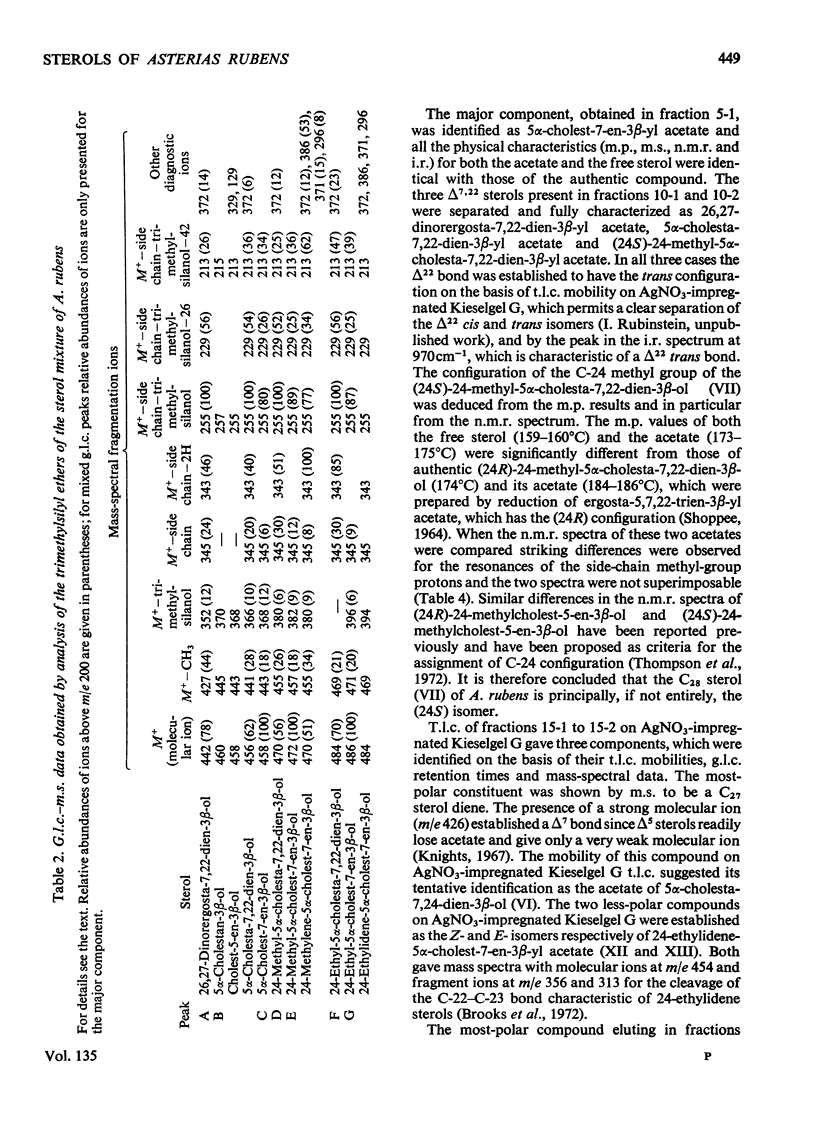
1. Twenty-two sterols were identified in the starfish Asterias rubens (Phylum, Echinodermata; Class, Asteroidea). 2. The major 4-demethyl sterols had a Δ7 bond and the C27 compound 5α-cholest-7-en-3β-ol predominated over other mono- and di-unsaturated sterols belonging to the C26, C27, C28 and C29 series. 3. Small amounts of cholest-5-en-3β-ol and 5α-cholestan-3β-ol were also present. 4. The minor sterols identified all contained either one or two methyl groups at C-4 and are considered to be potential biosynthetic precursors of 5α-cholest-7-en-3β-ol. 5. Three sterols possessing a 9β,19-cyclopropane ring were also isolated and were probably derived by the starfish from a dietary source.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton D. H., Harrison D. M., Moss G. P., Widdowson D. A. Investigations on the biosynthesis of steroids and terpenoids. II. Role of 24-methylene derivatives in the biosynthesis of steroids and terpenoids. J Chem Soc Perkin 1. 1970;6:773–785. [PubMed] [Google Scholar]
- Barton D. H., Kempe U. M., Widdowson D. A. Investigations on the biosynthesis of steroids and terpenoids. VI. The sterols of yeast. J Chem Soc Perkin 1. 1972;4:513–522. doi: 10.1039/p19720000513. [DOI] [PubMed] [Google Scholar]
- Brooks C. J., Horning E. C., Young J. S. Characterization of sterols by gas chromatography-mass spectrometry of the trimethylsilyl ethers. Lipids. 1968 Sep;3(5):391–402. doi: 10.1007/BF02531277. [DOI] [PubMed] [Google Scholar]
- Brooks C. J., Knights B. A., Sucrow W., Radüchel B. The characterisation of 24-ethylidene-sterols. Steroids. 1972 Oct;20(4):487–497. doi: 10.1016/0039-128x(72)90045-1. [DOI] [PubMed] [Google Scholar]
- ENEROTH P., HELLSTROEM K., RYHAGE R. IDENTIFICATION AND QUANTIFICATION OF NEUTRAL FECAL STEROIDS BY GAS-LIQUID CHROMATOGRAPHY AND MASS SPECTROMETRY: STUDIES OF HUMAN EXCRETION DURING TWO DIETARY REGIMENS. J Lipid Res. 1964 Apr;5:245–262. [PubMed] [Google Scholar]
- Eneroth P., Hellström K., Ryhage R. Identification of two neutral metabolites of stigmasterol found in human feces. Bile acids and sterols I62. Steroids. 1965 Dec;6(6):707–720. doi: 10.1016/0039-128x(65)90095-4. [DOI] [PubMed] [Google Scholar]
- FAGERLUND U. H., IDLER D. R. Marine sterols. 6. Sterol biosynthesis in molluscs and echinoderms. Can J Biochem Physiol. 1960 Sep;38:997–1002. [PubMed] [Google Scholar]
- Galli G., Maroni S. Mass spectrometric investigations of some unsaturated sterols biosynthetically related to cholesterol. Steroids. 1967 Sep;10(3):189–197. doi: 10.1016/0039-128x(67)90046-3. [DOI] [PubMed] [Google Scholar]
- Gibbons G. F., Goad L. J., Goodwin T. W., Nes W. R. Concerning the role of lanosterol and cycloartenol in steroid biosynthesis. J Biol Chem. 1971 Jun 25;246(12):3967–3976. [PubMed] [Google Scholar]
- Goad L. J., Goodwin T. W. Studies on phytosterol biosynthesis: the sterols of Larix decidua leaves. Eur J Biochem. 1967 May;1(3):357–362. doi: 10.1007/978-3-662-25813-2_49. [DOI] [PubMed] [Google Scholar]
- Goad L. J., Goodwin T. W. The biosynthesis of sterols in higher plants. Biochem J. 1966 Jun;99(3):735–746. doi: 10.1042/bj0990735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L. J., Rubinstein I., Smith A. G. The sterols of echinoderms. Proc R Soc Lond B Biol Sci. 1972 Feb 15;180(1059):223–246. doi: 10.1098/rspb.1972.0016. [DOI] [PubMed] [Google Scholar]
- Goad L. J., Williams B. L., Goodwin T. W. Studies on phytosterol biosynthesis. The presence of 4-alpha,14-alpha-dimethyl-delta-8,24(28)-ergostadien-3-beta-ol in grapefruit peel and its co-occurrece with cycloeucalenol in higher plant tissues. Eur J Biochem. 1967 Dec;3(2):232–236. doi: 10.1111/j.1432-1033.1967.tb19521.x. [DOI] [PubMed] [Google Scholar]
- Gustafsson B. E., Gustafsson J. A., Sjövall J. Intestinal and fecal sterols in germfree and conventional rats. Bile acids and steroids 172. Acta Chem Scand. 1966;20(7):1827–1835. doi: 10.3891/acta.chem.scand.20-1827. [DOI] [PubMed] [Google Scholar]
- Lenfant M., Zissmann E., Lederer E. Biosynthesis of the ethyl side chain of stigmasterol derivatives by the slime mould Dictyostelium discoideum. Tetrahedron Lett. 1967 Mar;12:1049–1052. doi: 10.1016/s0040-4039(00)90634-8. [DOI] [PubMed] [Google Scholar]
- Lenton J. R., Hall J., Smith A. R., Ghisalberti E. L., Rees H. H., Goad L. J., Goodwin T. W. The utilization of potential phytosterol precursors by Ochromonas malhamensis. Arch Biochem Biophys. 1971 Apr;143(2):664–674. doi: 10.1016/0003-9861(71)90248-7. [DOI] [PubMed] [Google Scholar]
- MOORE P. R., BAUMANN C. A. Skin sterols. I. Colorimetric determination of cholesterol and other sterols in skin. J Biol Chem. 1952 Apr;195(2):615–621. [PubMed] [Google Scholar]
- MORRIS L. J. FRACTIONATION OF CHOLESTEROL ESTERS BY THIN-LAYER CHROMATOGRAPHY. J Lipid Res. 1963 Jul;4:357–359. [PubMed] [Google Scholar]
- OHTA G., SHIMIZU M. Studies on the constituents of rice bran oil. II. Structure of oryzanol-A. Pharm Bull. 1957 Feb;5(1):40–44. doi: 10.1248/cpb1953.5.40. [DOI] [PubMed] [Google Scholar]
- Rahman R., Sharpless K. B., Spencer T. A., Clayton R. B. Removal of the 4,4-dimethyl carbons in the enzymic conversion of lanosterol to cholesterol. Initial loss of the 4-alpha-methyl group. J Biol Chem. 1970 May 25;245(10):2667–2671. [PubMed] [Google Scholar]
- SAKAI K., TSUDA K. [Studies on steroids. XXIX. Experiments on the synthesis of cholesta-7,22-dien-3beta-ol]. Chem Pharm Bull (Tokyo) 1963 Apr;11:529–530. doi: 10.1248/cpb.11.529. [DOI] [PubMed] [Google Scholar]
- Sanghvi A., Balasubramanian D., Moscowitz A. On the beta configuration of the C-4 methyl in a 4-methyl-delta-8,24-cholestadien-3-beta-ol isolated from rat skin. Biochemistry. 1967 Mar;6(3):869–872. doi: 10.1021/bi00855a030. [DOI] [PubMed] [Google Scholar]
- Scallen T. J., Dhar A. K., Loughran E. D. Isolation and characterization of C-4 methyl intermediates in cholesterol biosynthesis after treatment of rat liver in vitro with cholestan-3 beta, 5 alpha,6 beta-triol. J Biol Chem. 1971 May 25;246(10):3168–3174. [PubMed] [Google Scholar]
- Scallen T. J., Krueger W. Nuclear magnetic resonance and infrared spectra of delta-24- and C-24 saturated steroids. J Lipid Res. 1968 Jan;9(1):120–128. [PubMed] [Google Scholar]
- Schroepfer G. J., Jr, Lutsky B. N., Martin J. A., Huntoon S., Fourcans B., Lee W. H., Vermilion J. Recent investigations on the nature of sterol intermediates in the biosynthesis of cholesterol. Proc R Soc Lond B Biol Sci. 1972 Feb 15;180(1059):125–146. [PubMed] [Google Scholar]
- Smith A. G., Goad L. J. Sterol biosynthesis in the starfish Asterias rubens and Henricia sanguinolenta. Biochem J. 1971 Jul;123(4):671–673. doi: 10.1042/bj1230671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. G., Goad L. J. The metabolism of cholesterol by the echinoderms Aterias rubens and Solaster papposus. FEBS Lett. 1971 Jan 25;12(4):233–235. doi: 10.1016/0014-5793(71)80028-5. [DOI] [PubMed] [Google Scholar]
- Smith A. G., Goodfellow R., Goad L. J. The intermediacy of 3-oxo steroids in the conversion of cholest-5-en-3 -ol into 5 -cholestan-3 -ol by the starfish Asterias rubens and Porania pulvillus. Biochem J. 1972 Aug;128(5):1371–1372. doi: 10.1042/bj1281371. [DOI] [PMC free article] [PubMed] [Google Scholar]


