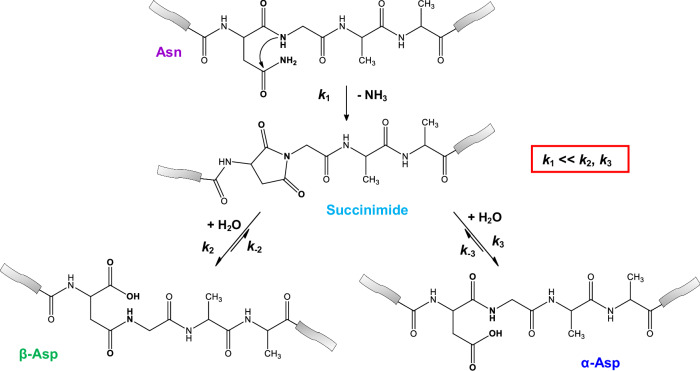
Fig. 1. A typical isomerisation of Asn-Gly sequence motif.
The isomerisation of the Asn-Gly sequence motif proceeds by N-nucleophilic attack of the glycine N-atom on the asparagine side chain carbonyl carbon, resulting in the formation of a five-membered succinimide followed by the release of ammonia. Subsequent hydrolysis leads to two different products, a segment containing β-Asp and a segment containing α-Asp. On the one hand, the side chain amide -CONH2 is replaced by a -COOH group and, on the other hand, the β-Asp nonproteinogenic residue appears within the primary sequence, in which the backbone is extended but the side chain is reduced by a -CH2- group with respect to the α-Asp residue.