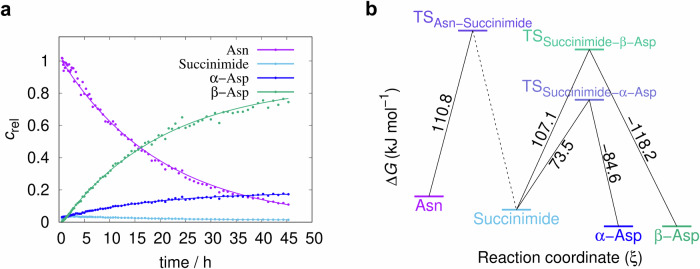
Fig. 2. The time course of the AsnGly isomerisation reaction and the resulting activation energies.
a The decomposition of -α-NGAA- via isomerisation and the formation of the products β-Asp (β-DGAA) and α-Asp (α-DGAA), respectively (pH=7.4, T = 46 °C), via the intermediate succinimide. The vertical axis is scaled with arbitrary units proportional to selected non-overlapping NMR signal integrals plotted as a function of the time. b ΔG‡ profile of the -α-NGAA- isomerisation reaction at pH 7.4, based on selected non-overlapping NMR signal integral data from 1H-NMR experiments.