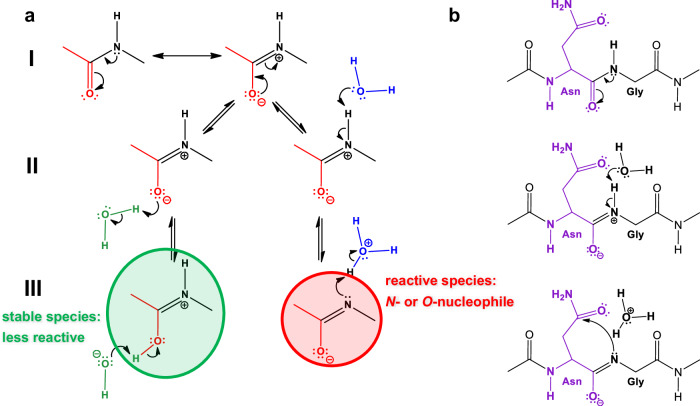
Fig. 3. The isomerisation reaction requires the formation of an N-nucleophile.
a First, the two non-equivalent resonance hybrids of the amide bond (a/I) must be considered. Secondly, the imino form, the zwitterion, can bind a water molecule in two different ways (a/II). The left one can be more stable (marked in green), while the right one is more reactive, producing an N- and O-nucleophile (marked in red) (a/III). b A way to explain how the Asn-Gly isomerisation is initiated using the latter type of nucleophile, which is simply formed with the aid of a water molecule (b/II). The ring closure of the Asn-Gly subunit (b/III) to form succinimide, shown as the rate-limiting step of the isomerisation reaction.