Abstract
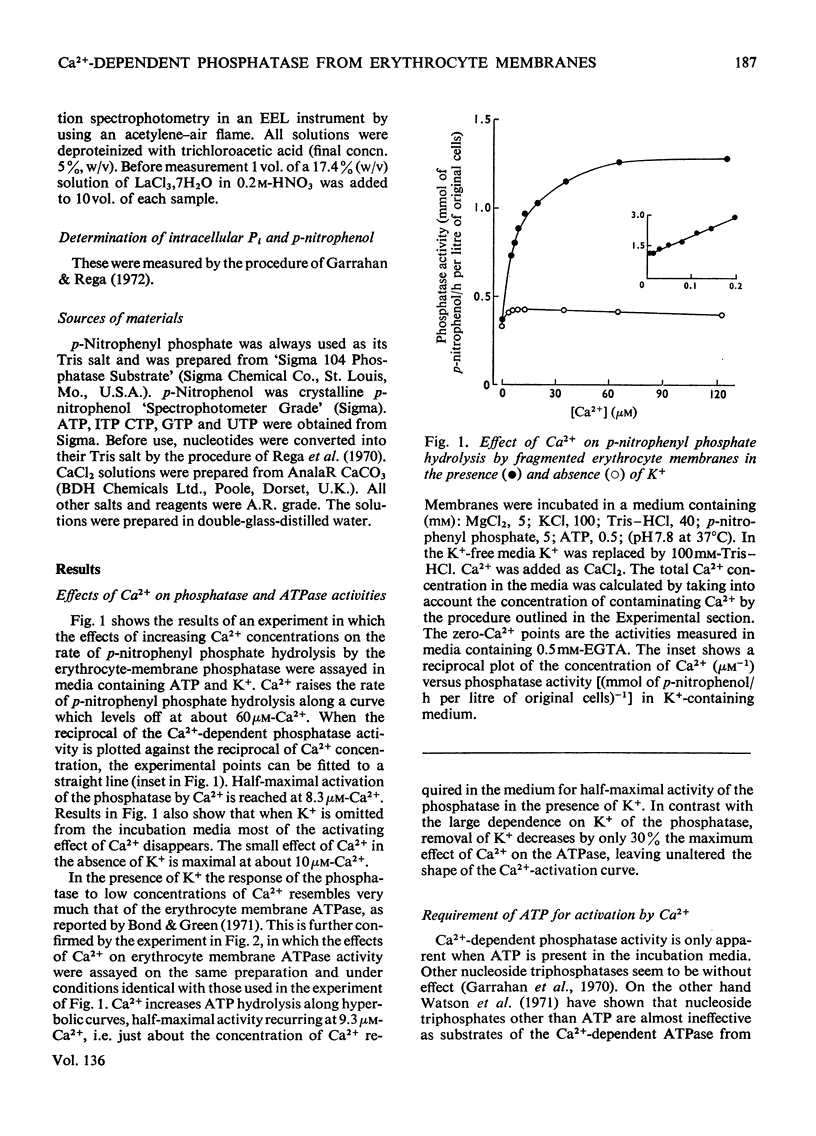
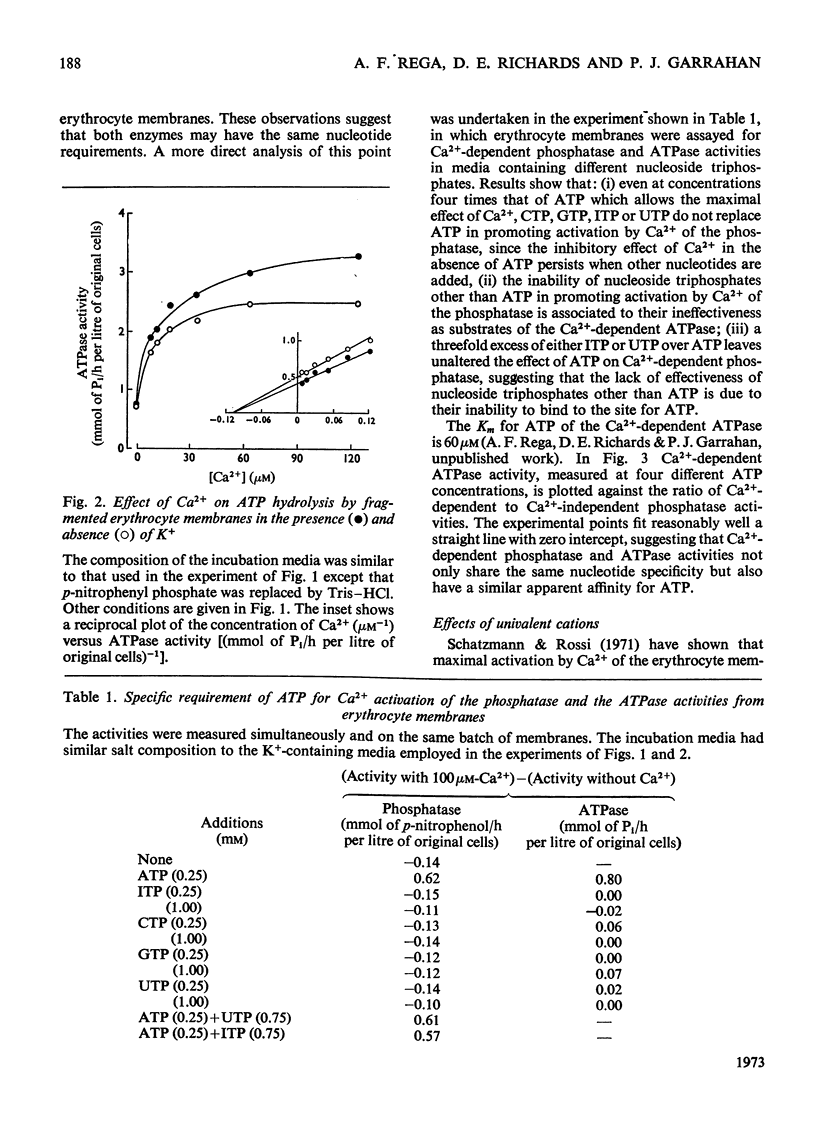
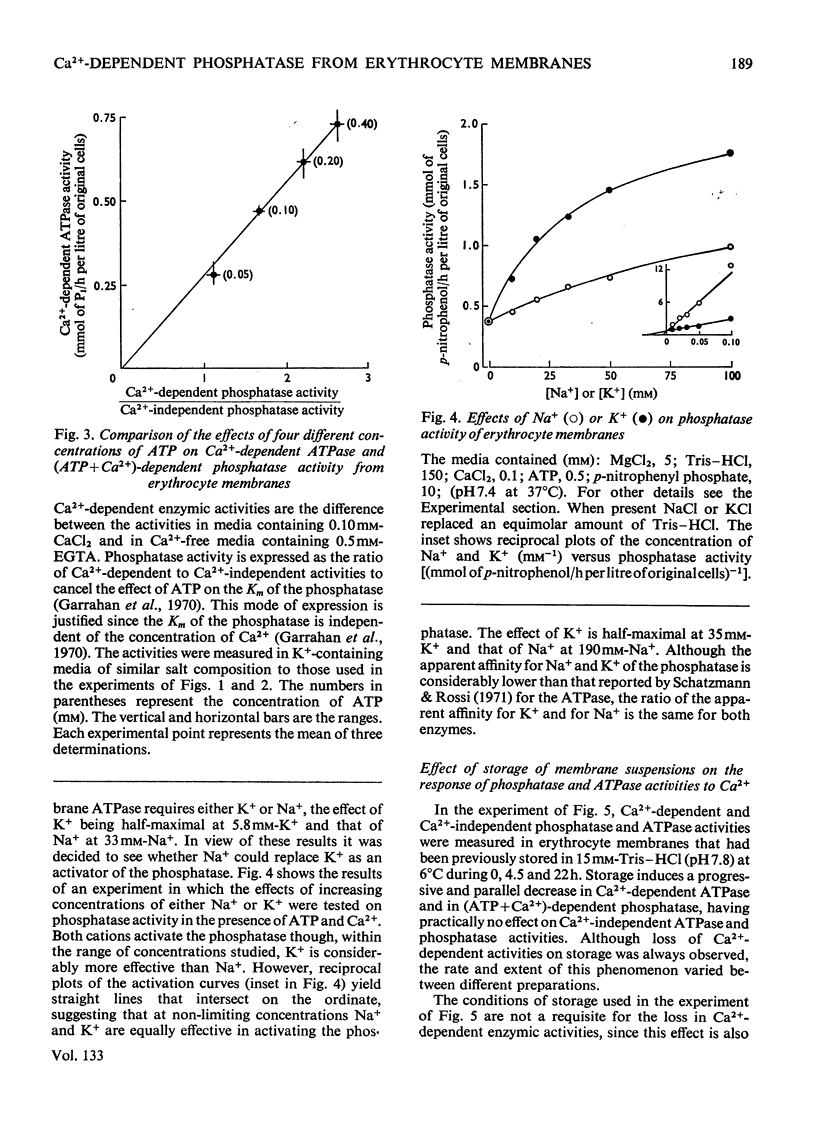
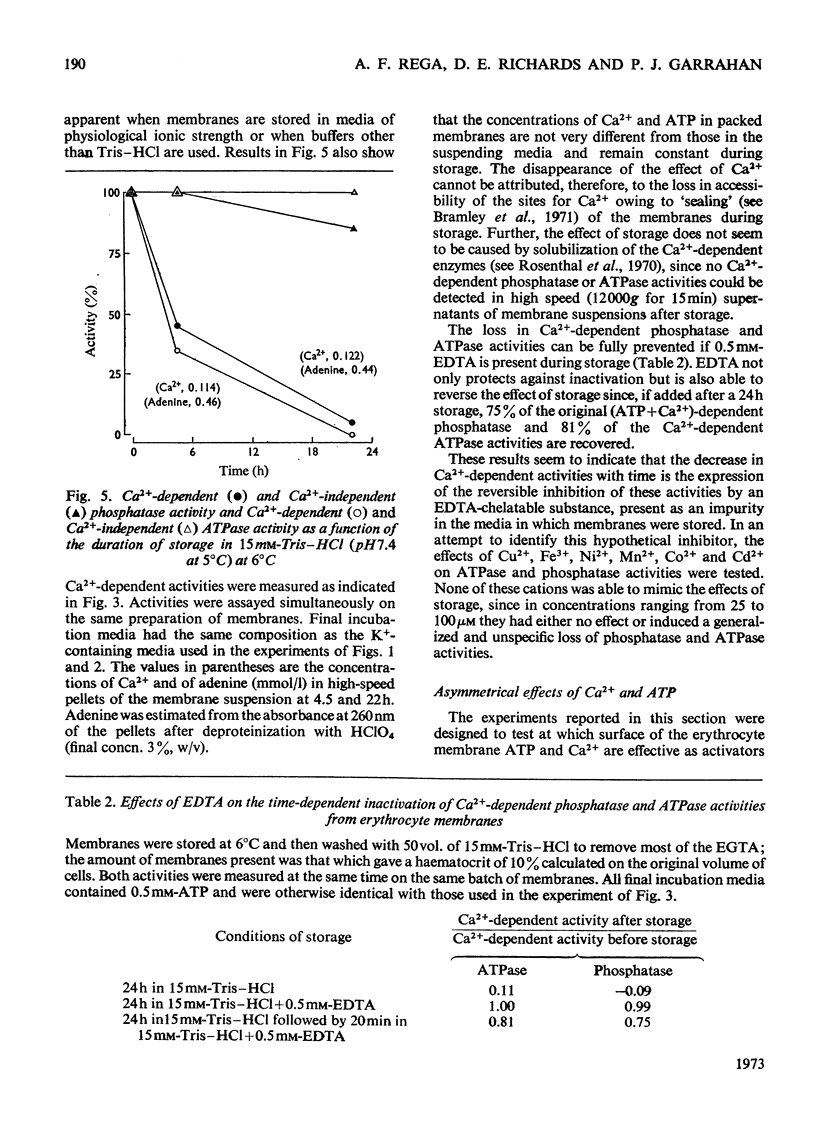
In the presence of ATP and of Mg2+, human erythrocyte membranes show a phosphatase activity towards p-nitrophenyl phosphate which is activated by low concentrations of Ca2+. The effect of Ca2+ is strongly enhanced if either K+ or Na+ is also present. Activation of the p-nitrophenyl phosphate phosphatase by Ca2+ reaches a half-maximum at about 8μm-Ca2+ and is apparent only when the ion has access to the inner surface of the cell membrane. Ca2+-dependent phosphatase activity can only be observed if ATP is at the inner surface of the cell membrane, and the presence of ATP seems to be absolutely necessary, since either its removal or its replacement by other nucleoside triphosphates abolishes the activating effect of Ca2+. The properties of the (ATP+Ca2+)-dependent phosphatase are very similar to those of the Ca2+-dependent ATPase (adenosine triphosphatase), also present in erythrocyte membranes, which probably is involved in Ca2+ transport in erythrocytes. The similarities suggest that both activities may be properties of the same molecular system. This view is further supported by the fact that p-nitrophenyl phosphate inhibits to a similar extent Ca2+-dependent ATPase activity and ATP-dependent Ca2+ extrusion from erythrocytes.
Full text
PDF


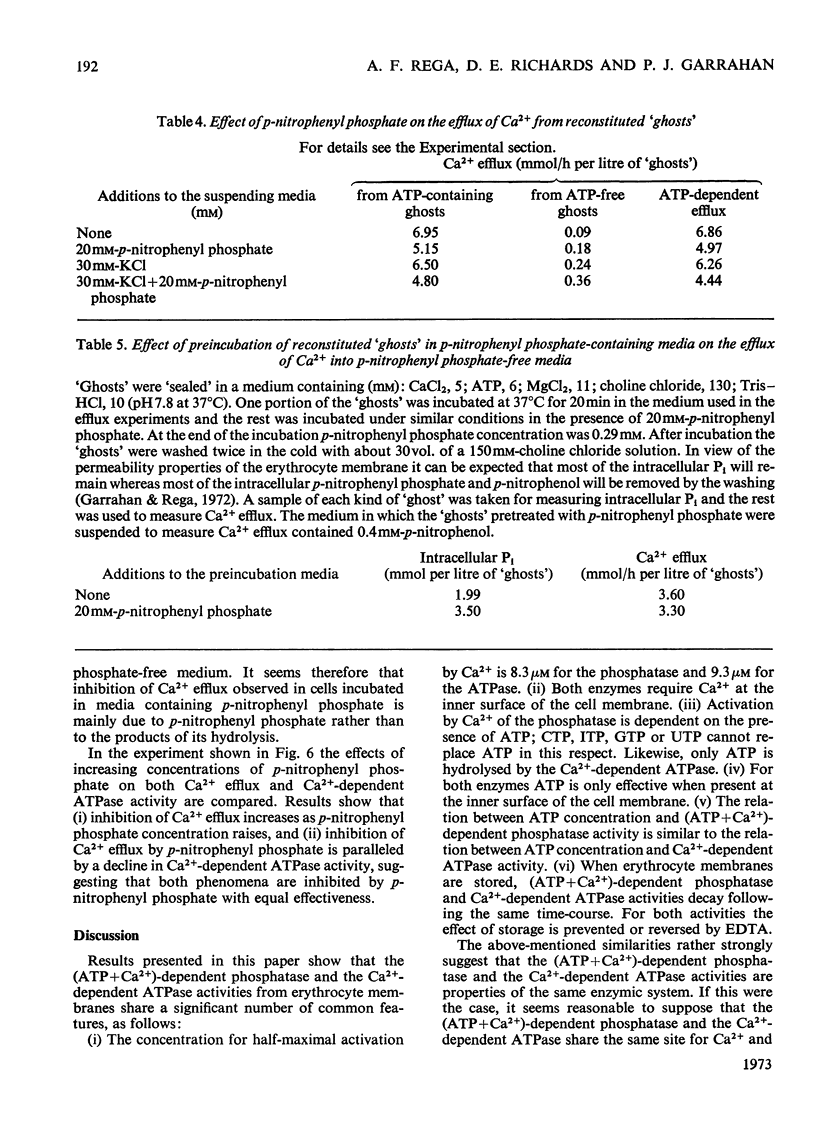
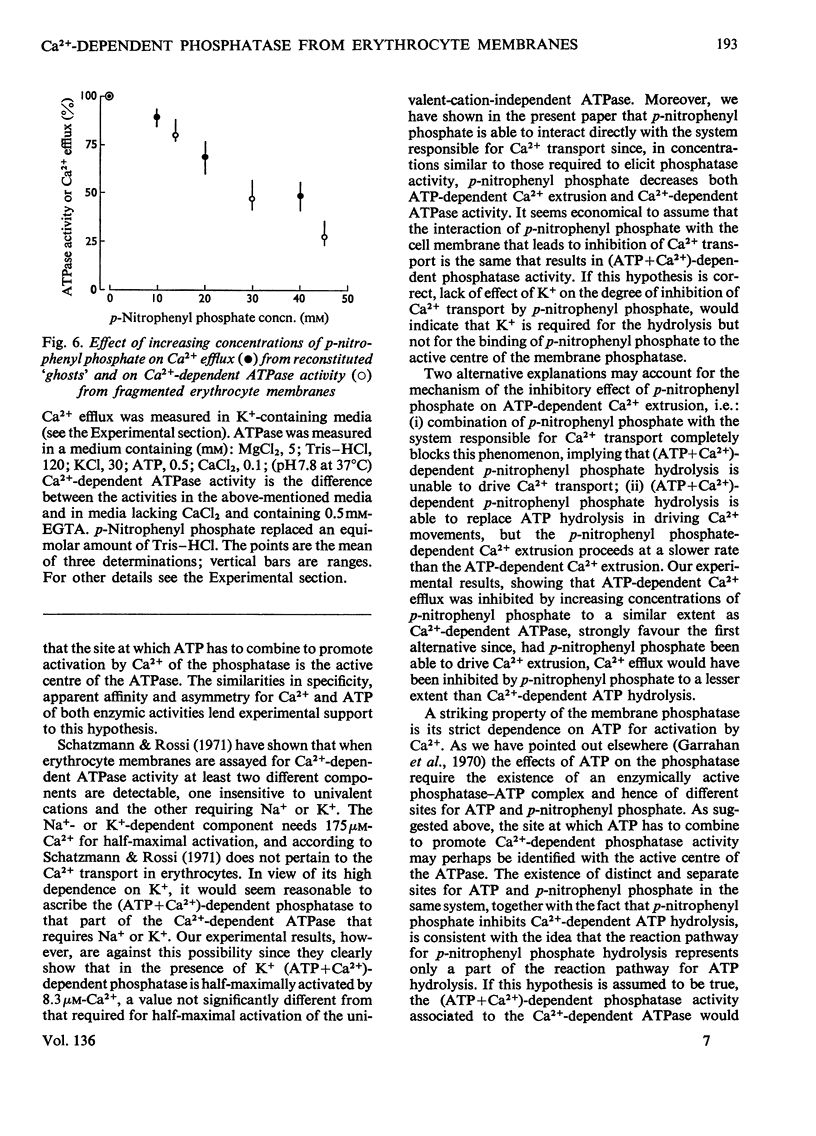

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond G. H., Green J. W. Effects of monovalent cations on the (Mg 2+ + Ca 2+ )-dependent ATPase of the red cell membrane. Biochim Biophys Acta. 1971 Aug 13;241(2):393–398. doi: 10.1016/0005-2736(71)90038-1. [DOI] [PubMed] [Google Scholar]
- Bramley T. A., Coleman R., Finean J. B. Chemical, enzymological and permeability properties of human erythrocyte ghosts prepared by hypotonic lysis in media of different osmolarities. Biochim Biophys Acta. 1971 Sep 14;241(3):752–769. doi: 10.1016/0005-2736(71)90003-4. [DOI] [PubMed] [Google Scholar]
- DUNHAM E. T., GLYNN I. M. Adenosinetriphosphatase activity and the active movements of alkali metal ions. J Physiol. 1961 Apr;156:274–293. doi: 10.1113/jphysiol.1961.sp006675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Pouchan M. I., Rega A. F. Potassium activated phosphatase from human red blood cells. The mechanism of potassium activation. J Physiol. 1969 Jun;202(2):305–327. doi: 10.1113/jphysiol.1969.sp008813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrahan P. J., Rega A. F. Potassium activated phosphatase from human red blood cells. The effects of p-nitrophenylphosphate on carbon fluxes. J Physiol. 1972 Jun;223(2):595–617. doi: 10.1113/jphysiol.1972.sp009864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Hoffman J. F., Lew V. L. Some "partial reactions" of the sodium pump. Philos Trans R Soc Lond B Biol Sci. 1971 Aug 20;262(842):91–102. doi: 10.1098/rstb.1971.0080. [DOI] [PubMed] [Google Scholar]
- Harrison D. G., Long C. The calcium content of human erythrocytes. J Physiol. 1968 Dec;199(2):367–381. doi: 10.1113/jphysiol.1968.sp008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G. p-nitrophenyl phosphate hydrolysis and calcium ion transport in fragmented sarcoplasmic reticulum. Science. 1971 Mar 5;171(3974):901–903. doi: 10.1126/science.171.3974.901. [DOI] [PubMed] [Google Scholar]
- Pouchan M. I., Garrahan P. J., Rega A. F. Effects of ATP and Ca2+ on a K+-activated phosphatase from red blood cell membranes. Biochim Biophys Acta. 1969 Jan 28;173(1):151–154. doi: 10.1016/0005-2736(69)90048-0. [DOI] [PubMed] [Google Scholar]
- Rega A. F., Garrahan P. J., Wainer S. R. Asymmetrical activation by Ca2+ of the erythrocyte membrane K+ -dependent phosphatase. Experientia. 1972 Oct 15;28(10):1158–1159. doi: 10.1007/BF01946142. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Kregenow F. M., Moses H. L. Some characteristics of a Ca2+ dependent ATPase activity associated with a group of erythrocyte membrane proteins which form fibrils. Biochim Biophys Acta. 1970;196(2):254–262. doi: 10.1016/0005-2736(70)90013-1. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J., Rossi G. L. (Ca 2+ + Mg 2+ )-activated membrane ATPases in human red cells and their possible relations to cation transport. Biochim Biophys Acta. 1971 Aug 13;241(2):379–392. doi: 10.1016/0005-2736(71)90037-x. [DOI] [PubMed] [Google Scholar]
- Schatzmann H. J., Vincenzi F. F. Calcium movements across the membrane of human red cells. J Physiol. 1969 Apr;201(2):369–395. doi: 10.1113/jphysiol.1969.sp008761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E. L., Vincenzi F. F., Davis P. W. Nucleotides as substrates of Ca-ATPase and NaK-ATPase in isolated red cell membranes. Life Sci II. 1971 Dec 22;10(24):1399–1404. doi: 10.1016/0024-3205(71)90349-3. [DOI] [PubMed] [Google Scholar]
- Wins P., Schoffeniels E. Studies on red-cell ghost ATPase systems: properties of a (Mg2+ + Ca2+)-dependent ATPase. Biochim Biophys Acta. 1966 Jul 13;120(3):341–350. doi: 10.1016/0926-6585(66)90301-3. [DOI] [PubMed] [Google Scholar]


