Abstract
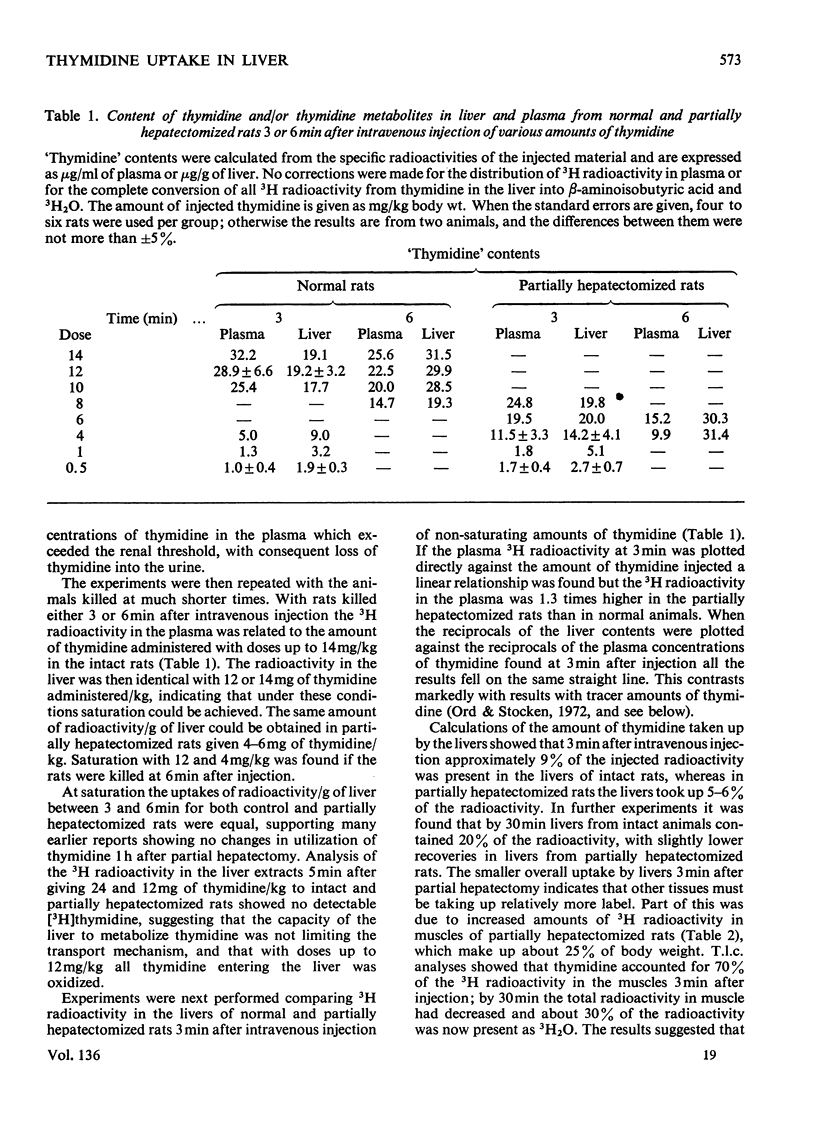
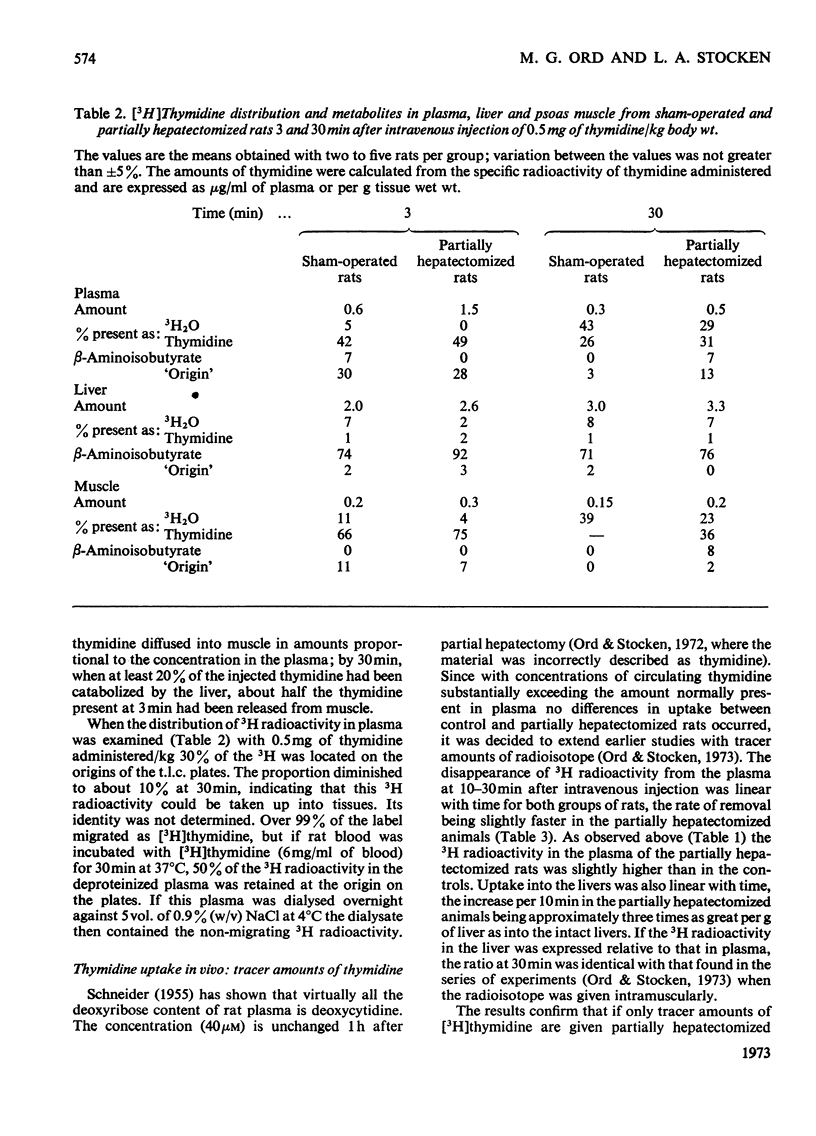
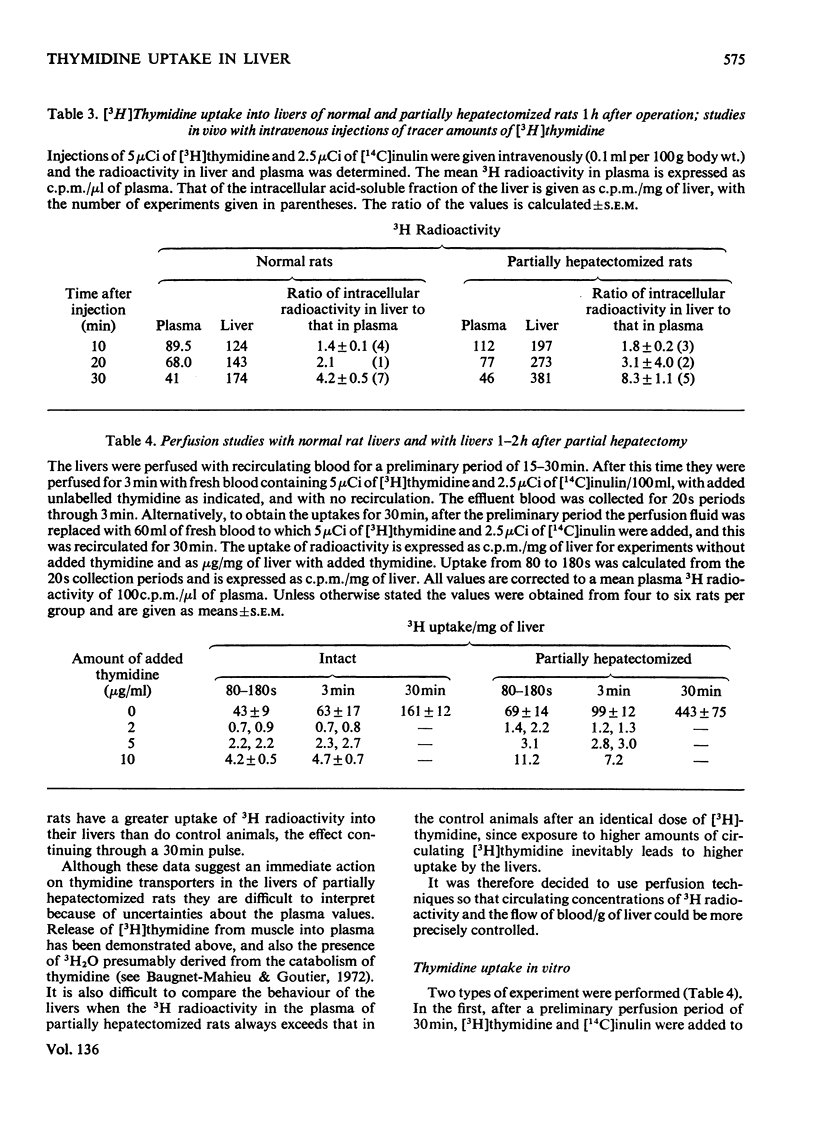
1. When [3H]thymidine was injected intravenously into rats in amounts up to 40mg/kg body wt. and the 3H radioactivity in the livers measured at 30min, saturation kinetics for thymidine uptake were not found. If the animals were examined 3 min after intravenous injection, saturation could be attained in normal rats with 12mg of thymidine/kg and in partially hepatectomized rats with 4mg/kg. At concentrations of thymidine close to saturation, no differences were found in rate or amount of uptake/g of liver between normal and partially hepatectomized rats 1–2h after operation. 2. Perfusion techniques were used to compare thymidine uptakes in the two sets of rats at concentrations up to 40μm-thymidine. Uptakes with tracer amounts of thymidine after 30min were identical in vivo and in the perfusion studies and were twice as great in livers from partially hepatectomized rats with concentrations up to 40μm-thymidine. 3. At 1.5h after operation there was nearly twice as much β-aminoisobutyrate present per g of liver from partially hepatectomized as compared with normal rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baugnet-Mahieu L., Goutier R. Reutilization of labelled-thymidine and -iododeoxyuridine for nuclear and mitochondrial DNA synthesis in regenerating rat liver. Arch Int Physiol Biochim. 1972 Apr;80(2):319–330. doi: 10.3109/13813457209075285. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Uptake of amino acids and nucleic acid precursors by regenerating rat liver. Biochem J. 1972 Aug;129(1):175–181. doi: 10.1042/bj1290175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ord M. G., Stocken L. A. Uptake of orotate and thymidine by normal and regenerating rat livers. Biochem J. 1973 Jan;132(1):47–54. doi: 10.1042/bj1320047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plagemann P. G., Roth M. F. Permeation as the rate-limiting step in the phosphorylation of uridine and choline and their incorporation into macromolecules by Novikoff hepatoma cells. Competitive inhibition by phenethyl alcohol, persantin, and adenosine. Biochemistry. 1969 Dec;8(12):4782–4789. doi: 10.1021/bi00840a020. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B., Tenenhouse A. The role of cyclic AMP and calcium in cell activation. CRC Crit Rev Biochem. 1972 Feb;1(1):95–148. doi: 10.3109/10409237209102545. [DOI] [PubMed] [Google Scholar]
- SCHNEIDER W. C. Deoxyribosides in animal tissues. J Biol Chem. 1955 Sep;216(1):287–301. [PubMed] [Google Scholar]
- SCHNEIDER W. C. Further studies on the assay of deoxyribosyl compounds in tissue extracts. J Biol Chem. 1962 May;237:1405–1409. [PubMed] [Google Scholar]
- Shimizu H., Daly J. W., Creveling C. R. A radioisotopic method for measuring the formation of adenosine 3',5'-cyclic monophosphate in incubated slices of brain. J Neurochem. 1969 Dec;16(12):1609–1619. doi: 10.1111/j.1471-4159.1969.tb10360.x. [DOI] [PubMed] [Google Scholar]
- Tolstoshev P., Wells J. R. The absence of saturated pyrimidine bases in chromatin-associated RNA from avian reticulocytes and mouse ascites cells. Biochem Biophys Res Commun. 1973 Mar 5;51(1):223–231. doi: 10.1016/0006-291x(73)90532-9. [DOI] [PubMed] [Google Scholar]


