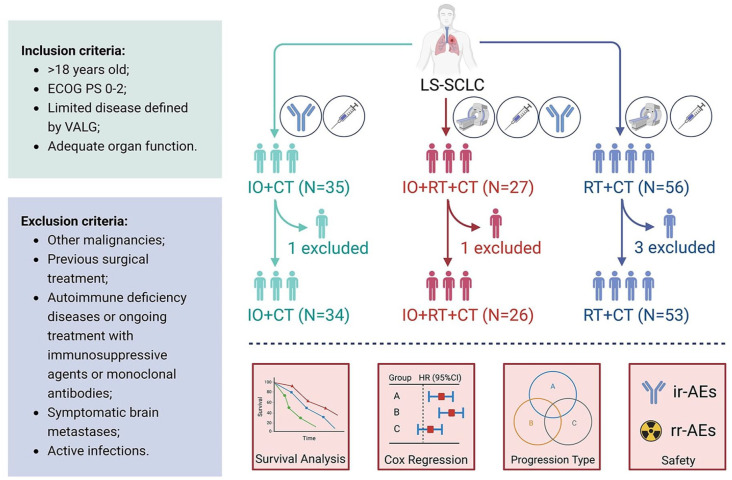
Figure 1.
Flow chart of the study design. Patients with LS-SCLC were screened according to established inclusion and exclusion criteria and divided into immune combined chemotherapy group (N = 34), immune combined chemoradiotherapy group (N = 26), and chemoradiotherapy group (N = 53) according to the treatment used. Survival information, prognostic factors, progression patterns, and safety of patients were also recorded and analyzed.
CT, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; IO, immunotherapy; ir-AE, immune-related adverse event; LS-SCLC, limited-stage small-cell lung cancer; rr-AE, radiation-related adverse event; RT, radiotherapy; VALG, Veterans Administration Lung Study Group.