Abstract
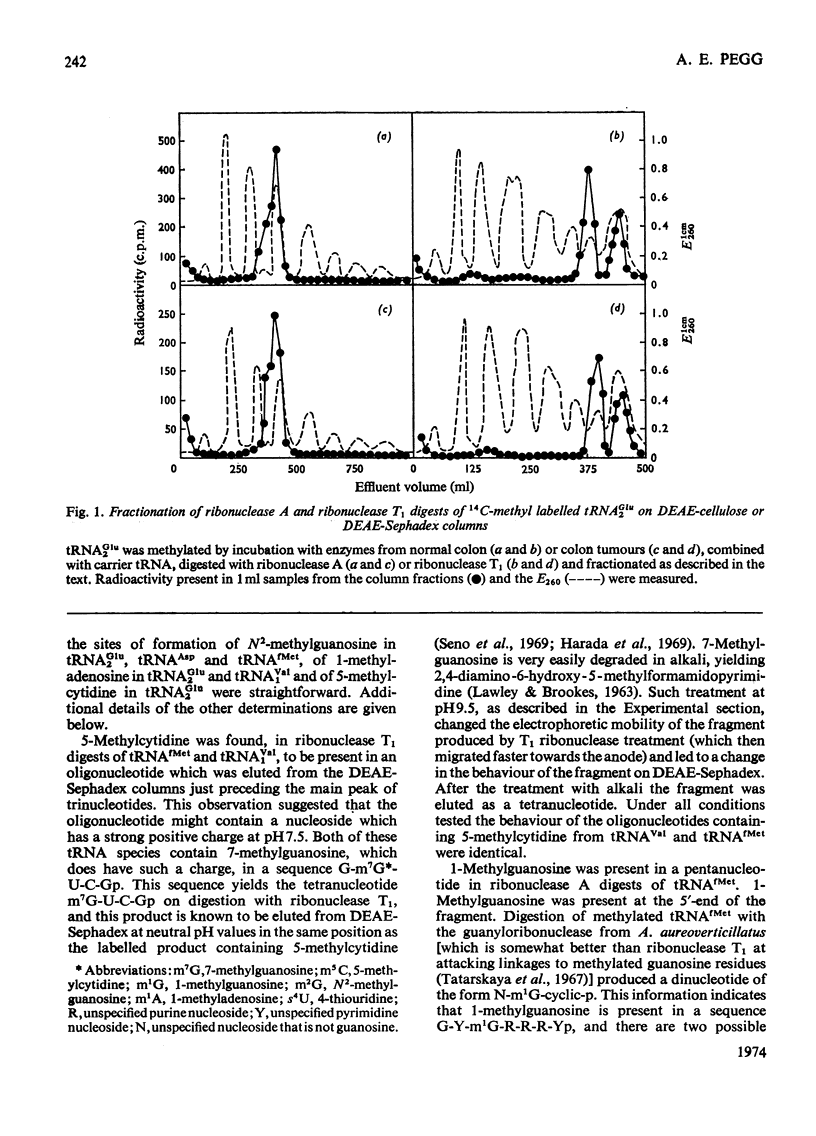
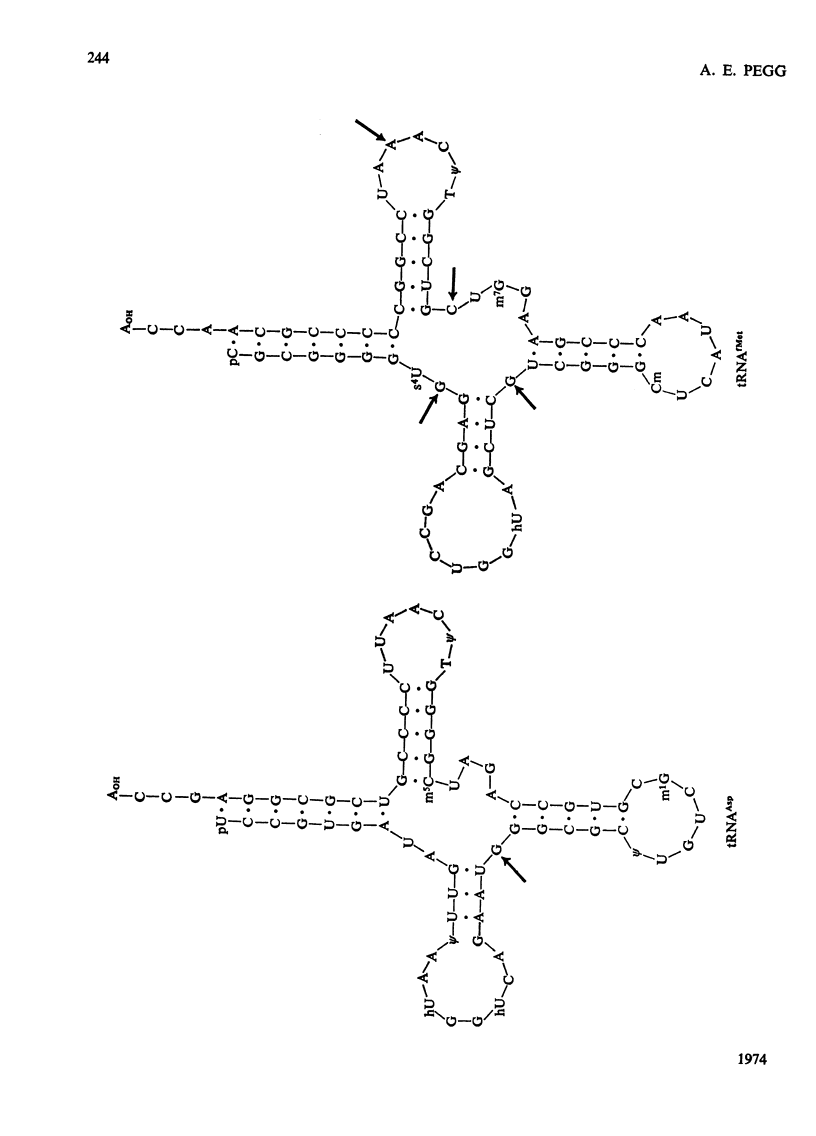
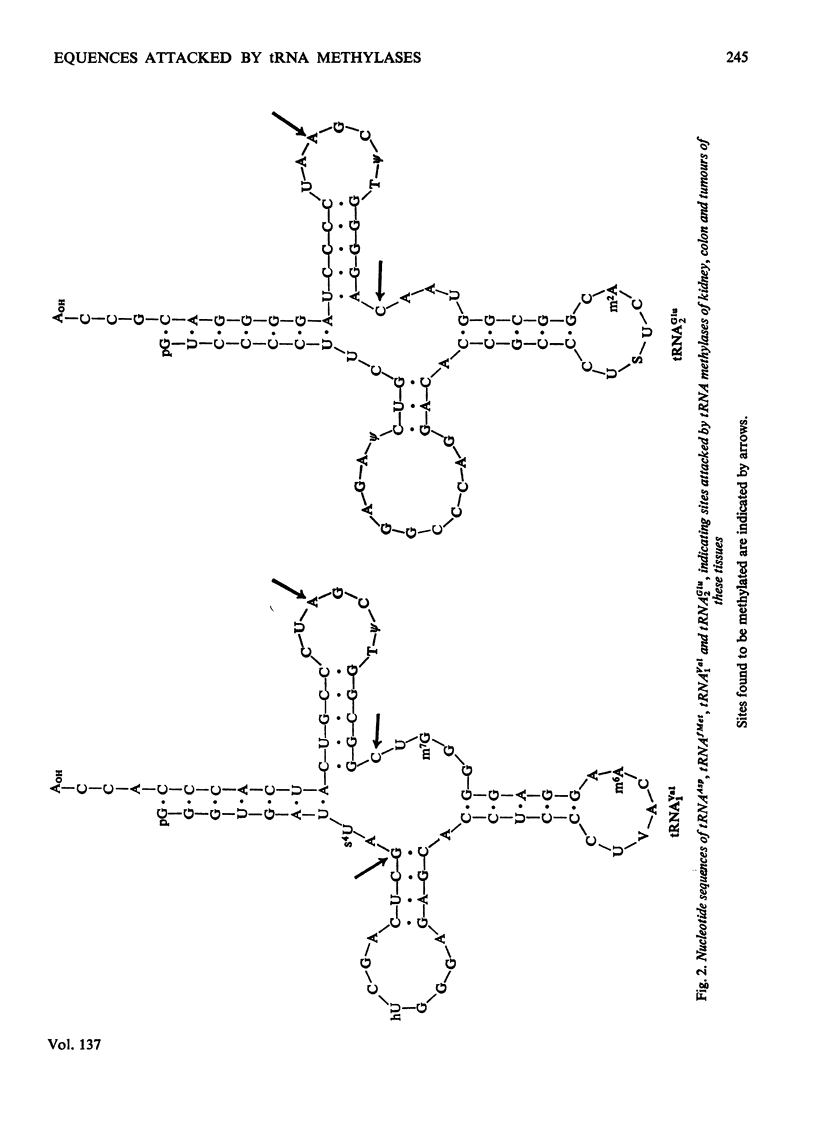
1. The sites within the tRNA sequence of nucleosides methylated by the action of enzymes from mouse colon, rat kidney and tumours of these tissues acting on tRNAAsp from yeast and on tRNAGlu2, tRNAfMet and tRNAVal1 from Escherichia coli were determined. 2. The same sites in a particular tRNA were methylated by all of these extracts. Thus tRNAGlu2 was methylated at the cytidine residue at position 48 and the adenosine residue at position 58 from the 5′-end of the molecule; tRNAAsp was methylated at the guanosine residue at position 26 from the 5′-end of the molecule; tRNAfMet was methylated at the guanosine residues 9 and 27, the cytidine residue 49 and the adenosine residue 59 from the 5′-end; tRNAVal1 was methylated at the guanosine residue 10, the cytidine residue 48 and the adenosine residue 58 from the 5′-end. 3. All of these sites within the clover leaf structure of the tRNA sequence are occupied by a methylated nucleoside in some tRNA species of known sequence. It is concluded that methylation of tRNA from micro-organisms by enzymes from mammalian tissues in vitro probably does accurately represent the specificity of these enzymes in vivo. However, there was no evidence that the tumour extracts, which had considerably greater tRNA methylase activity than the normal tissues, had methylases with altered specificity capable of methylating sites not methylated in the normal tissues.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Staehelin M. A comparison of 1-methyladenine-containing sequences in transfer ribonucleic acid from yeast and from rat liver. Biochemistry. 1969 Jan;8(1):257–262. doi: 10.1021/bi00829a036. [DOI] [PubMed] [Google Scholar]
- Baguley B. C., Staehelin M. Substrate specificity of adenine-specific transfer RNA methylase in normal and leukemic tissues. Eur J Biochem. 1968 Oct 17;6(1):1–7. doi: 10.1111/j.1432-1033.1968.tb00411.x. [DOI] [PubMed] [Google Scholar]
- Baguley B. C., Staehelin M. The specificity of transfer ribonucleic acid methylases from rat liver. Biochemistry. 1968 Jan;7(1):45–50. doi: 10.1021/bi00841a007. [DOI] [PubMed] [Google Scholar]
- Baguley B. C., Wehrli W., Staehelin M. In vitro methylation of yeast serine transfer ribonucleic acid. Biochemistry. 1970 Mar 31;9(7):1645–1649. doi: 10.1021/bi00809a026. [DOI] [PubMed] [Google Scholar]
- Borek E., Kerr S. J. Atypical transfer RNA's and their origin in neoplastic cells. Adv Cancer Res. 1972;15:163–190. doi: 10.1016/s0065-230x(08)60374-7. [DOI] [PubMed] [Google Scholar]
- Borek E. Transfer RNA and transfer RNA modification in differentiation and neoplasia. Introduction. Cancer Res. 1971 May;31(5):596–597. [PubMed] [Google Scholar]
- Burdon R. H. Ribonucleic acid maturation in animal cells. Prog Nucleic Acid Res Mol Biol. 1971;11:33–79. doi: 10.1016/s0079-6603(08)60325-6. [DOI] [PubMed] [Google Scholar]
- Craddock V. M. Transfer RNA methylases and cancer. Nature. 1970 Dec 26;228(5278):1264–1268. doi: 10.1038/2281264a0. [DOI] [PubMed] [Google Scholar]
- Dirheimer G., Ebel J. P., Bonnet J., Gangloff J., Keith G., Krebs B., Kuntzel B., Roy A., Weissenbach J., Werner C. Structure primaire des tRN. Biochimie. 1972;54(2):127–144. doi: 10.1016/s0300-9084(72)80097-x. [DOI] [PubMed] [Google Scholar]
- Dube S. K., Marcker K. A., Clark B. F., Cory S. The nucleotide sequence of N-formyl-methionyl-transfer RNA. Products of complete digestion with ribonuclease T-1 and pancreatic ribonuclease and derivation of their sequences. Eur J Biochem. 1969 Mar;8(2):244–255. doi: 10.1111/j.1432-1033.1969.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Gangloff J., Keith G., Dirheimer G. Méthode rapide de séparation et d'anaylyse d'un mélange d'oligonucléotides. Bull Soc Chim Biol (Paris) 1970 Mar 31;52(2):125–133. [PubMed] [Google Scholar]
- Gangloff J., Keith G., Ebel J. P., Dirheimer G. The primary structure of aspartate transfer ribonucleic acid from brewer's yeast. I. Complete digestion with pancreatic ribonuclease and T 1 ribonuclease. Biochim Biophys Acta. 1972 Jan 31;259(2):198–209. [PubMed] [Google Scholar]
- Gangloff J., Keith G., Ebel J. P., Dirheimer G. The primary structure of aspartate transfer ribonucleic acid from brewer's yeast. II. Partial digestions with pancreatic ribonuclease and T 1 ribonuclease and derivation of complete sequence. Biochim Biophys Acta. 1972 Jan 31;259(2):210–222. [PubMed] [Google Scholar]
- Gefter M. L., Bikoff E. Studies on synthesis and modification of transfer RNA. Cancer Res. 1971 May;31(5):667–670. [PubMed] [Google Scholar]
- Harada F., Kimura F., Nishimura S. Nucleotide sequence of oligonucleotides derived from Escherichia coli valine transfer RNA by ribonuclease T1 digestion: comparison of the sequences neighboring 3'- and 5'-terminals and anticodon region of Escherichia coli valine transfer RNA with those of yeast valine transfer RNA. Biochim Biophys Acta. 1969 Jun 17;182(2):590–592. doi: 10.1016/0005-2787(69)90219-6. [DOI] [PubMed] [Google Scholar]
- Kerr S. J., Borek E. The tRNA methyltransferases. Adv Enzymol Relat Areas Mol Biol. 1972;36:1–27. doi: 10.1002/9780470122815.ch1. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Quigley G. J., Suddath F. L., McPherson A., Sneden D., Kim J. J., Weinzierl J., Rich A. Three-dimensional structure of yeast phenylalanine transfer RNA: folding of the polynucleotide chain. Science. 1973 Jan 19;179(4070):285–288. doi: 10.1126/science.179.4070.285. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Endo H., Nishimura S. Comparison of the specificity and extent of in vitro methylation by guanylate residue-specific transfer RNA methylases isolated from ascites hepatoma, 3'-methyl-4-dimethylaminoazobenzene-induced hepatoma, and normal rat liver. Cancer Res. 1972 Jun;32(6):1243–1250. [PubMed] [Google Scholar]
- Kuchino Y., Nishimura S. Nucleotide sequence specificities of guanylate residue-specific tRNA methylases from rat liver. Biochem Biophys Res Commun. 1970 Jul 27;40(2):306–313. doi: 10.1016/0006-291x(70)91010-7. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Seno T., Nishimura S. Fragmented E. coli methionine tRNA f as methyl acceptor for rat liver tRNA methylase: alteration of the site of methylation by the conformational change of tRNA structure resulting from fragmentation. Biochem Biophys Res Commun. 1971 May 7;43(3):476–483. doi: 10.1016/0006-291x(71)90638-3. [DOI] [PubMed] [Google Scholar]
- LAWLEY P. D., BROOKES P. FURTHER STUDIES ON THE ALKYLATION OF NUCLEIC ACIDS AND THEIR CONSTITUENT NUCLEOTIDES. Biochem J. 1963 Oct;89:127–138. doi: 10.1042/bj0890127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboy P. S. Influence of polyamines and salts on changing patterns of tRNA methylation. FEBS Lett. 1971 Aug 1;16(2):117–120. doi: 10.1016/0014-5793(71)80347-2. [DOI] [PubMed] [Google Scholar]
- Levitt M. Detailed molecular model for transfer ribonucleic acid. Nature. 1969 Nov 22;224(5221):759–763. doi: 10.1038/224759a0. [DOI] [PubMed] [Google Scholar]
- McLean A. E., Magee P. N. Increased renal carcinogenesis by dimethyl nitrosamine in protein deficient rats. Br J Exp Pathol. 1970 Dec;51(6):587–590. [PMC free article] [PubMed] [Google Scholar]
- Munninger K. O., Chang S. H. A fluorescent nucleoside from glutamic acid tRNA of Escherichia coli K 12. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1837–1842. doi: 10.1016/0006-291x(72)90059-9. [DOI] [PubMed] [Google Scholar]
- Nau F., Garbit F., Dubert J. M. Specificities of tRNA methylases from mouse liver and plasmocytoma. Biochim Biophys Acta. 1972 Aug 16;277(1):80–86. doi: 10.1016/0005-2787(72)90354-1. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Ohashi Ziro, Harada Fumio, Nishimura Susumu. Primary sequence of glutamic acid tRNA II from Escherichia coli. FEBS Lett. 1972 Feb 1;20(2):239–241. doi: 10.1016/0014-5793(72)80804-4. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Hawks A. M. Further investigation of the increased transfer ribonucleic acid methylase activity in tumours of the mouse colon. Biochem J. 1974 Feb;137(2):229–238. doi: 10.1042/bj1370229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E. Methylation of purified transfer RNA preparations by extracts derived from rat kidney and kidney tumours. Biochim Biophys Acta. 1972 Mar 24;262(3):283–289. doi: 10.1016/0005-2787(72)90265-1. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Methylation of yeast aspartic acid transfer RNA by rat liver extracts. FEBS Lett. 1972 May 15;22(3):339–342. doi: 10.1016/0014-5793(72)80265-5. [DOI] [PubMed] [Google Scholar]
- RUSHIZKY G. W., BARTOS E. M., SOBER H. A. CHROMATOGRAPHY OF MIXED OLIGONUCLEOTIDES ON DEAE-SEPHADEX. Biochemistry. 1964 May;3:626–629. doi: 10.1021/bi00893a005. [DOI] [PubMed] [Google Scholar]
- SRINIVASAN P. R., BOREK E. ENZYMATIC ALTERATION OF NUCLEIC ACID STRUCTURE. Science. 1964 Aug 7;145(3632):548–553. doi: 10.1126/science.145.3632.548. [DOI] [PubMed] [Google Scholar]
- Seno T., Kobayashi M., Nishimura S. Resotration of methionine-accepting and transformylation activity by combining oligonucleotide fragments derived from a ribonuclease T-1 digest of Escherichia coli tRNA-fMet. Biochim Biophys Acta. 1969 Jan 21;174(1):408–411. doi: 10.1016/0005-2787(69)90270-6. [DOI] [PubMed] [Google Scholar]
- Shershneva L. P., Venkstern T. V., Bayev A. A. A study of tRNA methylase action. FEBS Lett. 1973 Jan 15;29(2):132–134. doi: 10.1016/0014-5793(73)80543-5. [DOI] [PubMed] [Google Scholar]
- Shershneva L. P., Venkstern T. V., Bayev A. A. A study of tRNA methylases by the dissected molecule method. FEBS Lett. 1971 May 20;14(5):297–298. doi: 10.1016/0014-5793(71)80283-1. [DOI] [PubMed] [Google Scholar]
- Shershneva L. P., Venkstern T. V., Bayev A. A. A study of transfer RNA methylation. Biochim Biophys Acta. 1973 Jan 19;294(2):250–262. [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972 Oct 17;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]
- Srinivasan P. R., Borek E. Enzymatic alteration of macromolecular structure. Prog Nucleic Acid Res Mol Biol. 1966;5:157–189. doi: 10.1016/s0079-6603(08)60234-2. [DOI] [PubMed] [Google Scholar]
- Staehelin M. The primary structure of transfer ribonucleic acid. Experientia. 1971 Jan 15;27(1):1–11. doi: 10.1007/BF02137708. [DOI] [PubMed] [Google Scholar]
- Starr J. L., Sells B. H. Methylated ribonucleic acids. Physiol Rev. 1969 Jul;49(3):623–669. doi: 10.1152/physrev.1969.49.3.623. [DOI] [PubMed] [Google Scholar]
- Wintermeyer W., Zachau H. G. A specific chemical chain scission of tRNA at 7-methylguanosine. FEBS Lett. 1970 Dec;11(3):160–164. doi: 10.1016/0014-5793(70)80518-x. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Barrell B. G. Nucleotide sequence of E. coli B tRNA1-Val. Nature. 1969 Apr 19;222(5190):278–279. doi: 10.1038/222278a0. [DOI] [PubMed] [Google Scholar]


