Abstract
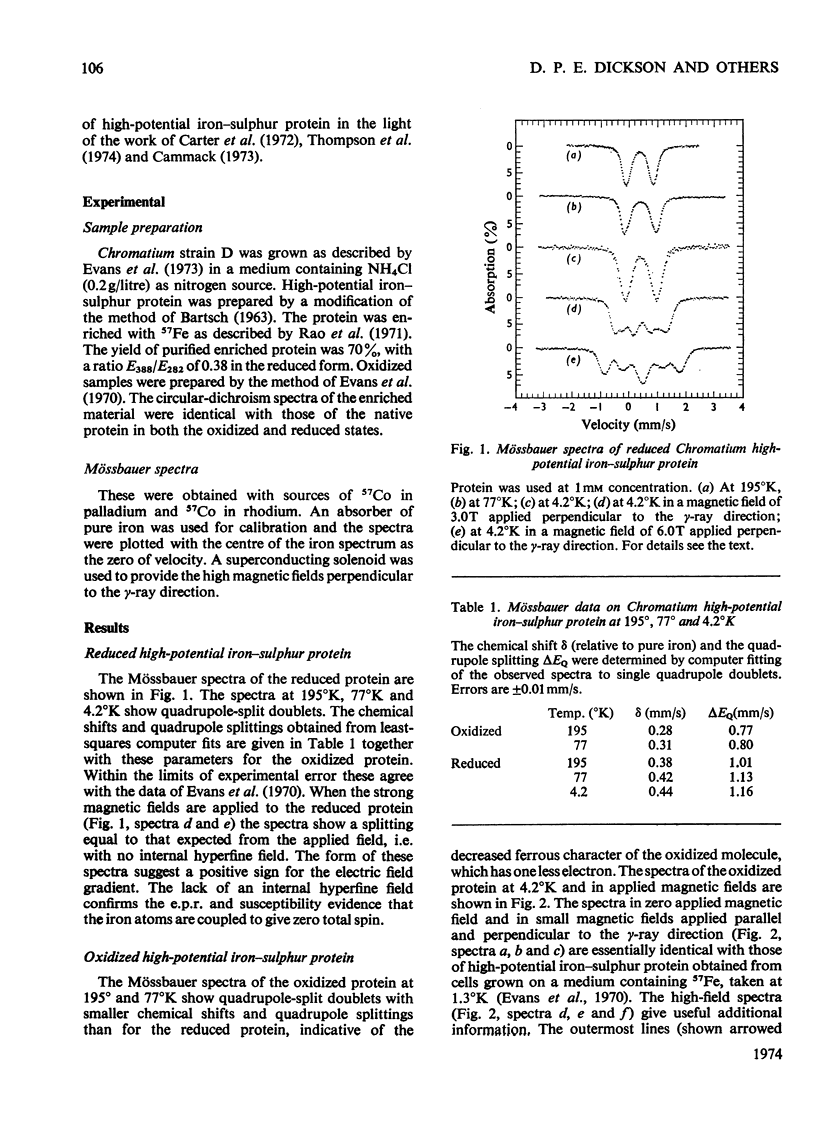
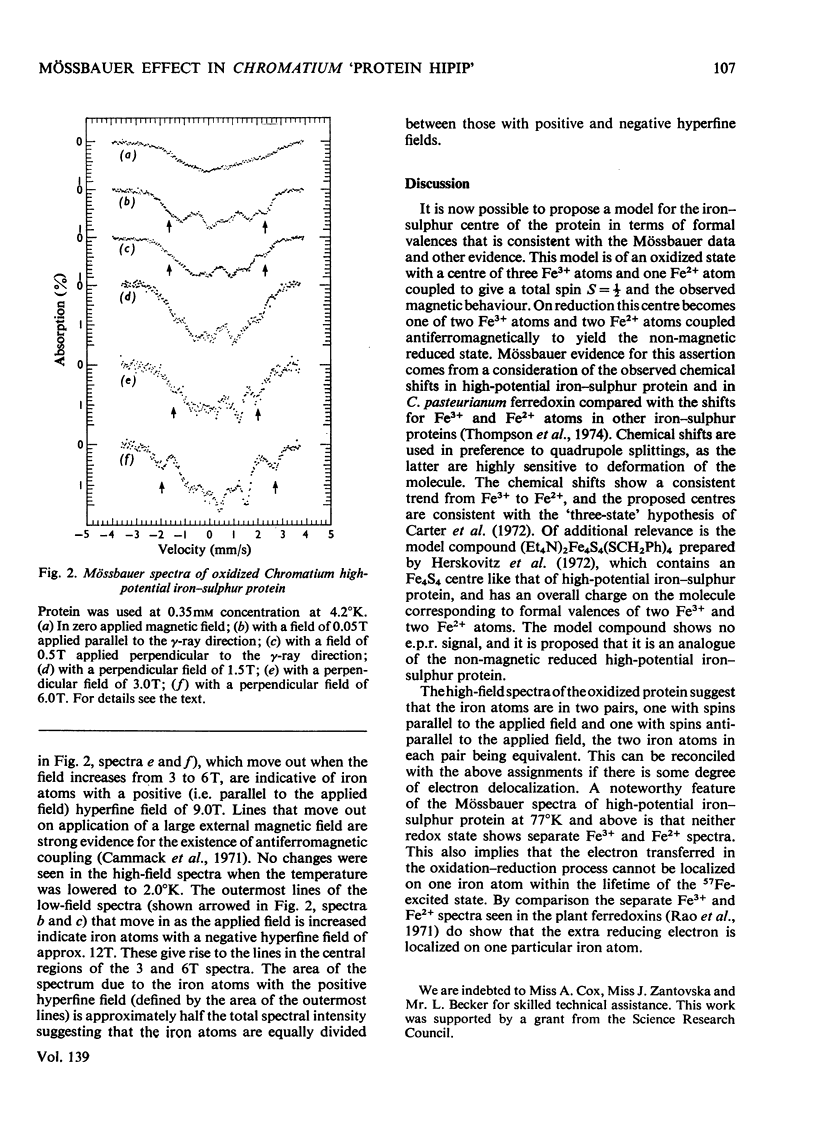
1. The previous Mössbauer work on Chromatium high-potential iron–sulphur protein by Moss et al. (1968) and Evans et al. (1970) was extended to high applied magnetic fields. 2. Measurements of the reduced protein confirm that it is non-magnetic. 3. Spectra of the oxidized protein in applied magnetic fields clearly indicate that some iron atoms have a positive hyperfine field, which is evidence for antiferromagnetic coupling. 4. The spectra can be interpreted in terms of two types of iron atom with positive and negative hyperfine fields of 9 and 12T respectively. 5. A consideration of the chemical shifts and other evidence suggests formal valences of two Fe3+ and two Fe2+ atoms in the non-magnetic reduced state, and three Fe3+ atoms and one Fe2+ atom in the oxidized state. 6. However, no separate Fe3+ and Fe2+ spectra are seen, suggesting that the d electrons are not localized on particular iron atoms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- Cammack R. "Super-reduction" of chromatium high-potential iron-sulphur protein in the presence of dimethyl sulphoxide. Biochem Biophys Res Commun. 1973 Sep 18;54(2):548–554. doi: 10.1016/0006-291x(73)91457-5. [DOI] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O., Johnson C. E. Mössbauer studies of adrenodoxin. The mechanism of electron transfer in a hydroxylase iron-sulphur protein. Biochem J. 1971 Dec;125(3):849–856. doi: 10.1042/bj1250849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. W., Jr, Freer S. T., Xuong N. H., Alden R. A., Kraut J. Structure of the iron-sulfur cluster in the Chromatius iron protein at 2.25 Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:381–385. doi: 10.1101/sqb.1972.036.01.049. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A., Sieker L. C., Adman E., Jensen L. H. A comparison of Fe 4 S 4 clusters in high-potential iron protein and in ferredoxin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3526–3529. doi: 10.1073/pnas.69.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dus K., De Klerk H., Sletten K., Bartsch R. G. Chemical characterization of high potential iron proteins from Chromatium and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1967 Jun 27;140(2):291–311. doi: 10.1016/0005-2795(67)90470-9. [DOI] [PubMed] [Google Scholar]
- Evans M. C., Hall D. O., Johnson C. E. Hyperfine structure of (57Fe) iron in the Mössbauer spectrum of the high-potential iron protein from Chromatium. Biochem J. 1970 Sep;119(2):289–291. doi: 10.1042/bj1190289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Telfer A., Smith R. V. The purification and some properties of the molybdenum-iron protein of Chromatium nitrogenase. Biochim Biophys Acta. 1973 Jun 15;310(2):344–352. doi: 10.1016/0005-2795(73)90114-1. [DOI] [PubMed] [Google Scholar]
- Herskovitz T., Averill B. A., Holm R. H., Ibers J. A., Phillips W. D., Weiher J. F. Structure and properties of a synthetic analogue of bacterial iron--sulfur proteins. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2437–2441. doi: 10.1073/pnas.69.9.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew S. G., Petering D., Palmer G., Foust G. P. Spectrophotometric titration of ferredoxins and Chromatium high potential iron protein with sodium dithionite. J Biol Chem. 1969 Jun 10;244(11):2830–2834. [PubMed] [Google Scholar]
- Moss T. H., Bearden A. J., Bartsch R. G., Cusanovich M. A., San Pietro A. Mössbauer spectroscopy of non-heme iron proteins. Biochemistry. 1968 Apr;7(4):1591–1596. doi: 10.1021/bi00844a048. [DOI] [PubMed] [Google Scholar]
- Moss T. H., Petering D., Palmer G. The magnetic susceptibility of oxidized and reduced ferredoxins from spinach and parsley and the high potential protein from Chromatium. J Biol Chem. 1969 May 10;244(9):2275–2277. [PubMed] [Google Scholar]
- Phillips W. D., Poe M., McDonald C. C., Bartsch R. G. Proton magnetic resonance studies of Chromatium high-potential iron protein. Proc Natl Acad Sci U S A. 1970 Oct;67(2):682–687. doi: 10.1073/pnas.67.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. L., Johnson C. E., Dickson D. P., Cammack R., Hall D. O., Weser U., Rao K. K. Mössbauer effect in the eight-iron ferredoxin from Clostridium pasterurianum. Evidence for the state of the iron atoms. Biochem J. 1974 Apr;139(1):97–103. doi: 10.1042/bj1390097. [DOI] [PMC free article] [PubMed] [Google Scholar]


