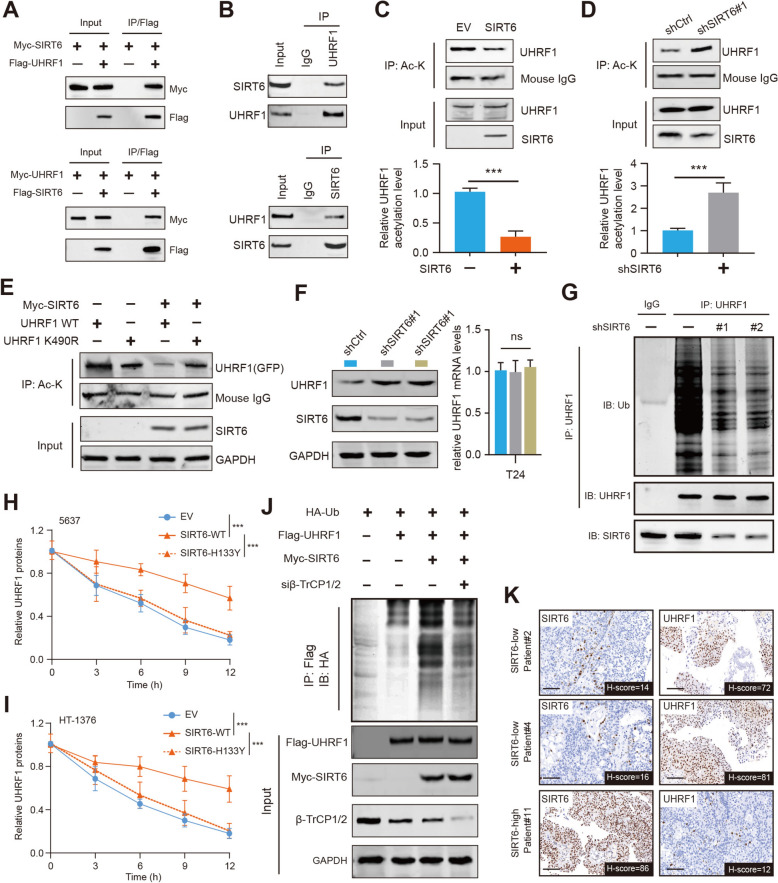
Fig. 3.
SIRT6 deacetylates UHRF1 and promotes β-TrCP1-mediated UHRF1 degradation. A Western blots of indicated proteins in WCL and co-IP samples of anti-FLAG antibody obtained from 293 T cells transfected with indicated plasmids. B The co-immunoprecipitation (co-IP) assay showing the endogenous interactions between SIRT6 and UHRF1 proteins. C Acetylation level of UHRF1 in 253J cells transfected with EV or SIRT6-OE plasmids probed with pan anti acetyl lysine antibodies. Lower is quantitative data showing a decreased level of UHRF1 acetylation in cells with SIRT6 OE. D Acetylation level of UHRF1 in T24 cells treated with shCtrl or shSIRT6 lentiviruses probed with pan anti acetyl lysine antibodies. Lower is quantitative data showing an increased level of UHRF1 acetylation in cells with SIRT6-KD. E 253J shUHRF1 cells overexpressing SIRT6 and UHRF1 WT or UHRF1 K490R were used to measure the acetylation level of UHRF1 at residue K490. F Western blotting and RT-qPCR assays detecting the UHRF1 expression levels in shCtrl and shSIRT6#1 BLCA cells. G Detection of UHRF1 ubiquitination by IP and IB as indicated in control and SIRT6-KD cells. H-I UHRF1 proteins in WCLs of 5637 (H) or HT-1376 cells (I) transfected with the indicated SIRT6 plasmids for 48 h and then treated with CHX (50 μg/ml) and harvested at different time points. J Ubiquitination levels of UHRF1 in cells treated with control or β-TrCP1 siRNAs as indicated. K The IHC and correlation analysis showing the reverse associations between SIRT6 and UHRF1 levels in BLCA samples. *p < 0.05, **p < 0.01, ***p < 0.001, ns no significance