Table 1. Optimization of the reaction conditionsa.
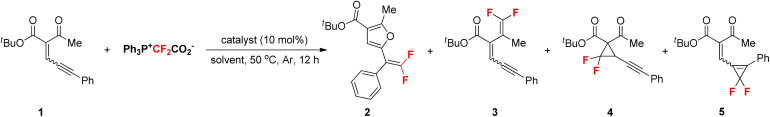
| |||
|---|---|---|---|
| Entry | Catalyst | Solvent | Yield of 2 (%) |
| 1 | None | 1,4-Dioxane | 45 |
| 2 | AlCl3 | 1,4-Dioxane | 45 |
| 3 | BF3 OEt2 | 1,4-Dioxane | 45 |
| 4 | HFIP | 1,4-Dioxane | 51 |
| 5 | PFTB | 1,4-Dioxane | 63 |
| 6 | PFTB | THF | 51 |
| 7 | PFTB | TBME | 63 |
| 8 | PFTB | CPME | 84 |
Reaction conditions: 1 (E/Z = 1 : 1, 0.1 mmol), Ph3P+CF2CO2− (2.0 equiv.), catalyst (10 mol%) in solvent (1.0 mL) at 50 °C under Ar for 12 h. 19F NMR yields with PhCF3 as internal standard. 3–5 were not found. PFTB = Perfluoro-tert-butanol; CPME = Cyclopentyl methyl ether; HFIP = 1,1,1,3,3,3-Hexafluoroisopropanol.
