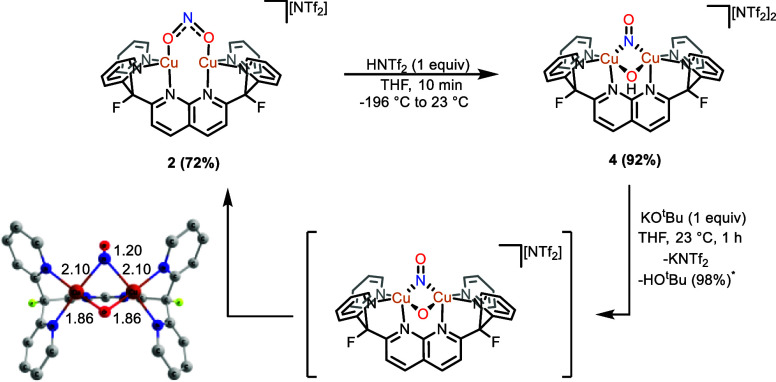
Figure 4.
Stoichiometric cycle illustrating the synthesis of [Cu2(μ-NO)(μ-OH)DPFN][NTf2]2 (4) and its deprotonation to yield [Cu2(μ-κ1:κ1-O2N)DPFN][NTf2] (2) via a dicopper(II,II) μ-NO, μ-O intermediate that undergoes N–O bond formation. The DFT-calculated geometry of [Cu2(μ-NO)(μ-O)DPFN]+ is also shown with hydrogens omitted for clarity. *Yield determined by integration of the 1H NMR spectrum against an internal standard.