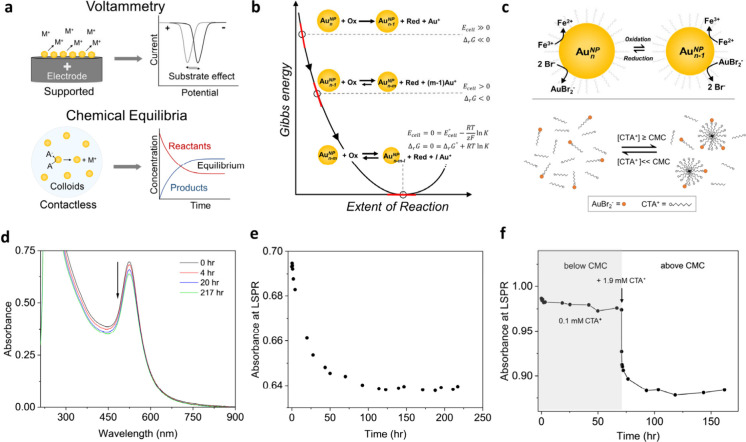
Figure 1.
Contactless method for determining standard reduction potentials (E0) of nanoparticles. (a) Comparison of the voltammetry method with our approach for determining reduction potentials of metallic nanoparticles. (b) Equilibrated reaction between gold nanoparticles and a redox couple for determining their E0. (c) Redox equilibrium of AuBr2–/AuNPn and Fe3+/2+ couple, and the complexation between CTA micelles and AuBr2–. (d and e) Time-lapsed UV–vis spectra (arrow indicates the reaction progress) and kinetic trace at plasmon resonance of a typical reaction between 10.9 nm nanoparticles and Fe3+. (f) Kinetic trace at the plasmon resonance of the reaction when the CTA+ concentration is increased from 0.1 to 1.9 mM.