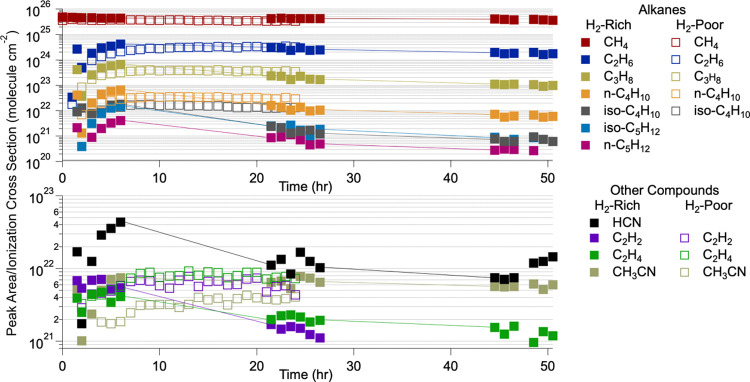
Figure 2.
Peak areas of each detected compound normalized to their electron ionization cross section at 70 eV as a function of the reaction time. Solid squares correspond to hydrogen-rich experiments, and open squares correspond to hydrogen-poor experiments. Alkanes are in the top panel, and the other compounds characterized during these experiments are in the bottom panel. The ionization cross section of n-C5H12 was used for iso-C5H12 as there is no reported electron ionization cross section for iso-C5H12.