Figure 1.
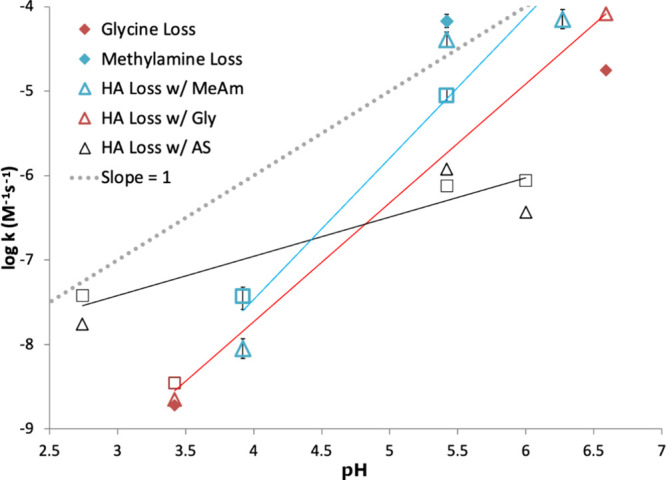
Measured room-temperature 2nd-order rate constants (M–1 s–1) as a function of pH, measured by 1H NMR for hydroxyacetone reactions with glycine (Gly, red), methylamine (MeAm, blue), and ammonium sulfate (AS, black), initial concentrations of all reactants = 0.5 M. Rate constants were calculated from initial loss rates of reactant signals, and the ±1σ error bars derived from uncertainties in fitted slopes shown for the methylamine reaction data set are typical for all three data sets. HA losses were followed at the CH3 signal (2.14 ppm, open triangles) and the CH2 signal (attached to the OH group, 4.4 ppm, open squares). HA losses with each reduced nitrogen species were fit with linear functions. Amine loss rate constants (filled diamonds) are shown for methylamine CH3 (2.58 ppm, blue) and glycine CH2 (3.55 ppm, red signals) when such losses were observable. AS losses could not be quantified by 1H NMR.
