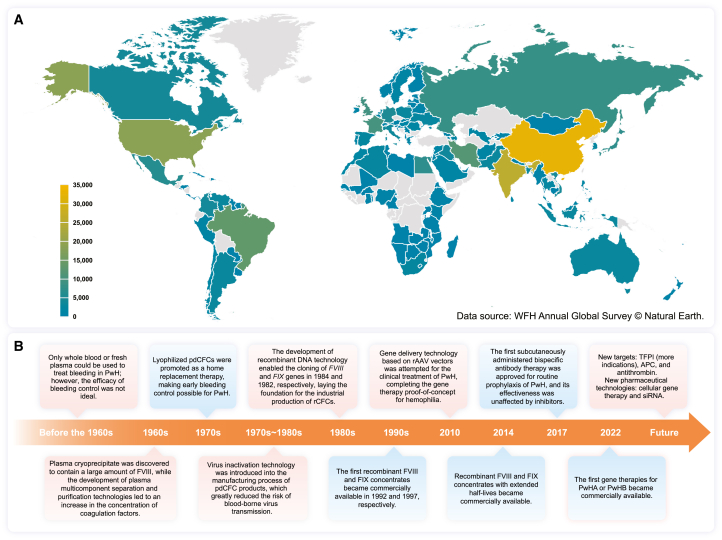
Figure 1.
Global distribution of registered PwH in 2022 and the historical timeline of biopharmaceutical products for hemophilia
(A) Total number of PwH in different countries or regions. Countries and regions with no reported data for 2022 are indicated in gray. Data source: WFH Annual Global Survey © Natural Earth. Abbreviations: PwH, patients with hemophilia; WFH, World Federation of Hemophilia.
(B) Advances in pharmaceutical technology driving the development of biopharmaceutical products for hemophilia. The red background indicates the introduction of biotechnology, whereas the blue background indicates the products. Abbreviations: PwH, patients with hemophilia; pdCFCs, plasma-derived coagulation factor concentrates; rCFCs, recombinant coagulation factor concentrates; rAAV, recombinant adeno-associated virus; TFPI, tissue factor pathway inhibitor; APC, activated protein C; siRNA, small interfering RNA.