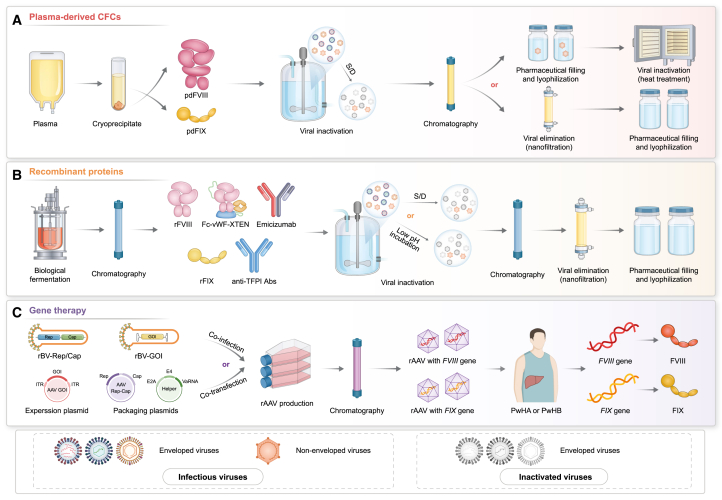
Figure 2.
Application of pharmaceutical technologies in drugs approved for hemophilia
(A) Healthy human plasma is the raw material from which blood factors (FVIII and FIX) are separated and purified. Abbreviations: pdFVIII, plasma-derived FVIII; pdFIX, plasma-derived FIX; S/D, solvent/detergent.
(B) Recombinant factors and monoclonal antibodies biosynthesized using recombinant DNA technology. Abbreviations: Abs, antibodies; rFVIII, recombinant FVIII; rFIX, recombinant FIX; S/D, solvent/detergent; TFPI, tissue factor pathway inhibitors.
(C) Viral gene therapies that introduce nucleic acids into patient somatic cells to correct disease-causing mutations. Abbreviations: Cap, capsid genes; GOI, gene of interest; ITR, inverted terminal repeat; PwHA, patients with hemophilia A; PwHB, patients with hemophilia B; rAAV, recombinant adeno-associated virus; rBV, recombinant baculovirus; Rep, replication genes.