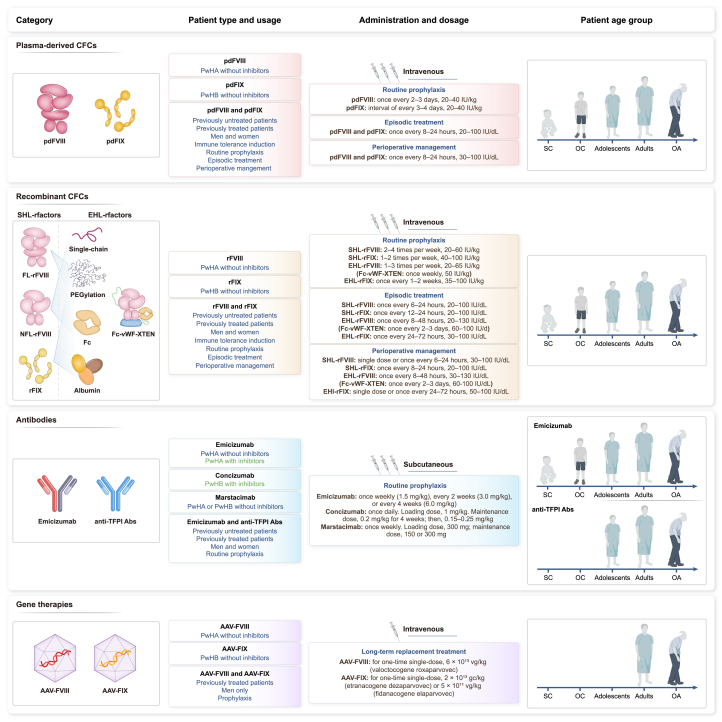
Figure 3.
Application scenarios for different biopharmaceutical products in patients with hemophilia
Approved biopharmaceutical products for hemophilia can be classified into three categories: coagulation factor concentrates (CFCs), recombinant monoclonal antibodies, and gene therapies. The products have different characteristics: blue font in the patient type and usage section indicates the common applications, and green indicates the unique advantages of the corresponding products. The half-life of EHL-FVIII and EHL-FIX is at least 1.3-fold and 3-fold greater than that of SHL-FVIII and SHL-FIX, respectively. Small children (SC): 0 to <6 years; older children (OC): 6 to <12 years; adolescents: 12 to <18 years; adults: 18 to <65 years; older adults (OA): ≥65 years. Abbreviations: Abs, antibodies; EHL, extended half-life; FL-rFVIII, full-length recombinant FVIII; gc, genome copies; NFL-rFVIII, non-full-length rFVIII; pdFVIII, plasma-derived FVIII; pdFIX, plasma-derived FIX; PwHA, patients with hemophilia A; PwHB, patients with hemophilia B; rFVIII, recombinant FVIII; SHL, standard half-life; TFPI, tissue factor pathway inhibitor; vg, vector genomes.