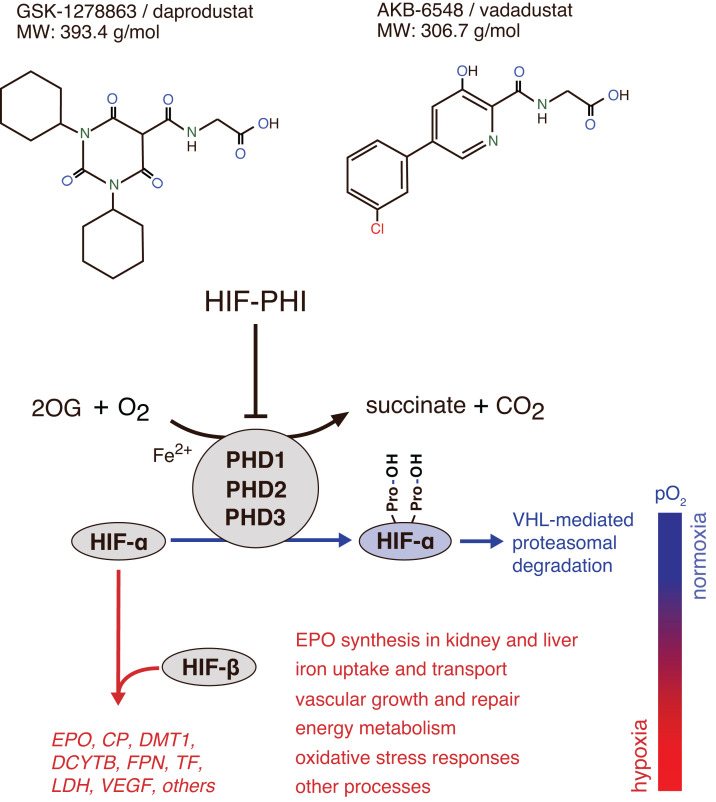
Figure 1.
Mechanisms of action of HIF-PHIs. Schematic overview of HIF activity regulation by PHD dioxygenases. The oxygen-sensitive HIF-α subunit is constitutively synthesized and rapidly degraded under normoxic conditions. Proteasomal degradation of HIF-α is initiated by prolyl-hydroxylation and mediated by the von Hippel-Lindau (VHL)-E3-ubiquitin ligase complex. PHD1, PHD2, and PHD3 utilize molecular oxygen and 2-oxoglutarate (2OG) for HIF-α hydroxylation. PHD2 is the main regulator of HIF activity in most cells. Hypoxia or exposure to HIF-PHIs reduces PHD catalytic activity, which results in intracellular accumulation of HIF-α and its nuclear translocation. In the nucleus, HIF-α heterodimerizes with constitutively expressed HIF-β, forming the HIF transcription factor, which increases the expression of HIF target genes such as EPO, ceruloplasmin (CP), divalent metal transporter 1 (DMT1), duodenal cytochrome b (DCYTB), ferroportin (FPN), transferrin (TF), lactate dehydrogenase (LDH), VEGF, and others. Also shown are examples of HIF-regulated biological processes. HIF-2 induces renal and hepatic EPO synthesis in response to hypoxia or HIF-PHI administration. Chemical structures of HIF-PHIs daprodustat (2-[(1,3-dicyclohexyl-2,4,6-trioxo-1,3-diazinane-5-carbonyl)amino]acetic acid) and vadadustat (2-[[5-(3-chlorophenyl)-3-hydroxypyridine-2-carbonyl]amino]acetic acid) are shown at the top. A common feature of HIF-PHIs is the presence of a carbonylglycine side chain, which is structurally analogous to 2-OG.