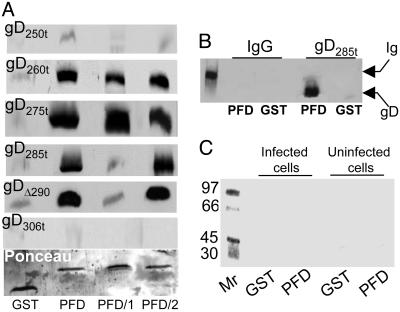
Fig. 4.
Binding of GST-PFD to soluble forms of gD (A) and lack of binding to infected and uninfected cell proteins (C) and unrelated protein (B). (A) Immunoblots of soluble forms of gD bound by GST-PFD fusion proteins. Each form of gD (40 ng) was reacted with GST or the chimeric proteins GST-PFD (PFD), GST-PFD/1 (PFD/1), or GST-PFD/2 (PFD/2), previously immobilized on GSH-beads. The beads were pelleted and extensively washed. The GSH-eluted proteins were separated by SDS/PAGE, blotted, and reacted with mAb H170. Blots were stained with Ponceau (Sigma) to control that equivalent amounts of GST fusion proteins were loaded (one example shown). (B) GST or GST-PFD (PFD) immobilized on GSH-beads was incubated with murine IgG or gD285t as positive control. The GSH-eluted proteins were reacted with mAb H170 and anti-mouse Ab. The leftmost lane shows the migration position of the Ig heavy chain. (C) Autoradiographic image of a gel showing proteins from uninfected or HSV-1(F)-infected cells bound to GST or GST-PFD (PFD). Vero cells were labeled with [35S]methionine and [35S]cysteine, solubilized with 1% Triton X-100, and absorbed to GST or GST-PFD, previously immobilized on GSH-beads. The GSH-eluted proteins were analyzed by autoradiography. The leftmost lane shows the migration positions of Mr markers.