TABLE 1.
Overview of clinical approved MET inhibitors for the treatment of NSCLC.
| Types of MET inhibitors | Drugs | Chemical structures | Phase | Clinical trial number | Status |
|---|---|---|---|---|---|
| Ia | Crizotinib (1) |
|
III |
NCT02767804 NCT04632758 NCT03052608 NCT06569420 NCT02838420 NCT02075840 |
Active |
| I/II |
NCT01712217 NCT01970865 |
Completed | |||
| IV | NCT03672643 | Recruiting | |||
| II |
NCT01945021 NCT02183870 NCT03088930 NCT00932451 |
Completed | |||
| III |
NCT06140836 NCT06082635 NCT04603807 |
Recruiting | |||
| I/II | NCT05384626 | Recruiting | |||
| II | NCT01500824 | Withdrawn | |||
| IV | NCT05160922 | Active | |||
| I |
NCT01579994 NCT01998126 |
Completed | |||
| I/II | NCT02584634 | Terminated | |||
| II | NCT04322578 | Recruiting | |||
| III |
NCT02737501 NCT01639001 NCT00932893 NCT01828112 |
Completed | |||
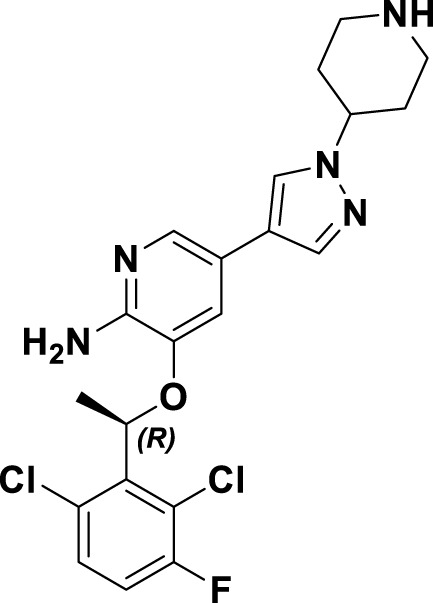
| Ib | Capmatinib (2) |
|
II |
NCT04460729 NCT05567055 NCT03240393 |
Withdrawn |
| II |
NCT02414139 NCT03647488 NCT02750215 NCT01610336 NCT02276027 |
Completed | |||
| II | NCT04677595 | Active | |||
| IV | NCT05110196 | Recruiting | |||
| I/II | NCT05488314 | Active | |||
| III | NCT04427072 | Completed | |||
| III | NCT04816214 | Terminated | |||
| I |
NCT01911507 NCT01324479 |
Completed | |||
| II |
NCT05642572 NCT03040973 |
Recruiting | |||
| I | NCT02468661 | Terminated | |||
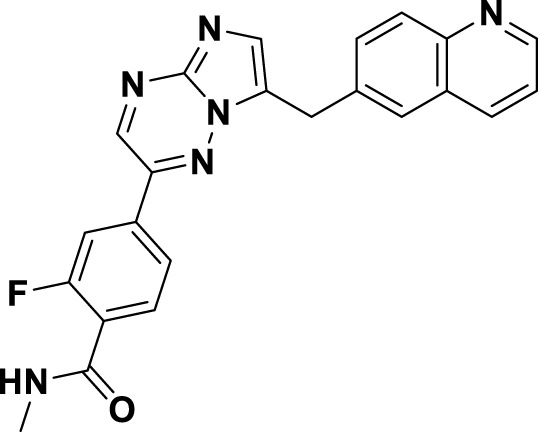
| Savolitinib (3) |
|
III | NCT04923945 | Recruiting | |
| II | NCT02117167 | Completed | |||
| II | NCT04322890 | Recruiting | |||
| II |
NCT03778229 NCT05163249 NCT03833440 NCT04606771 NCT03944772 |
Active | |||
| III | NCT05261399 | Recruiting | |||
| II | NCT04322578 | Recruiting | |||
| I | NCT02374645 | Completed | |||
| I | NCT02143466 | Active | |||
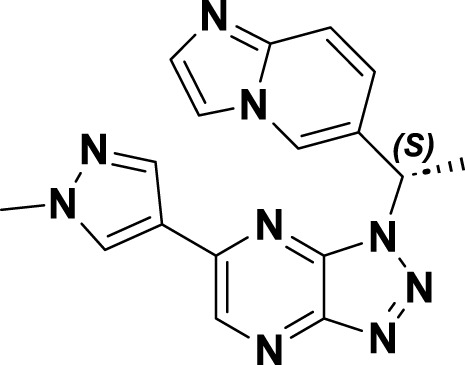
| SAR125844 (4) |
|
I | NCT01391533 | Completed | |
| I | NCT01657214 | Completed | |||
| II | NCT02435121 | Completed | |||
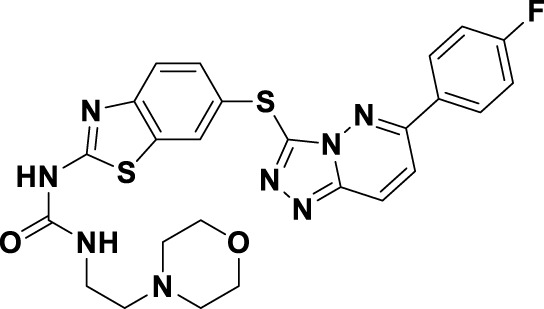
| Bozitinib (5) |
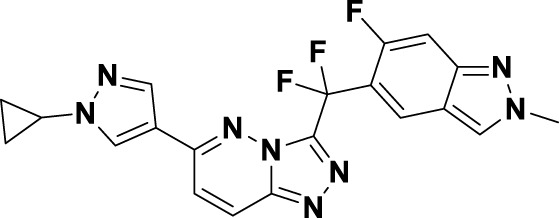
|
II | NCT03175224 | Recruiting | |
| I/II |
NCT04743505 NCT06343064 |
Recruiting | |||
| I | NCT02896231 | Completed | |||
| I/II | NCT03655613 | Terminated | |||
| Tepotinib (6) |
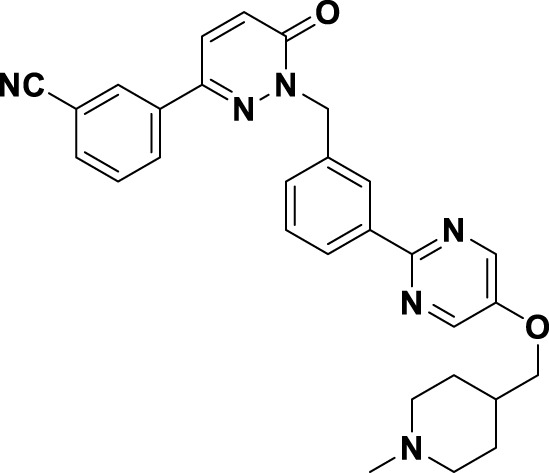
|
I/II | NCT04739358 | Terminated | |
| I/II | NCT06083857 | Recruiting | |||
| II |
NCT02864992 NCT03940703 |
Active | |||
| I/II | NCT01982955 | Completed | |||
| ABN401 (7) |
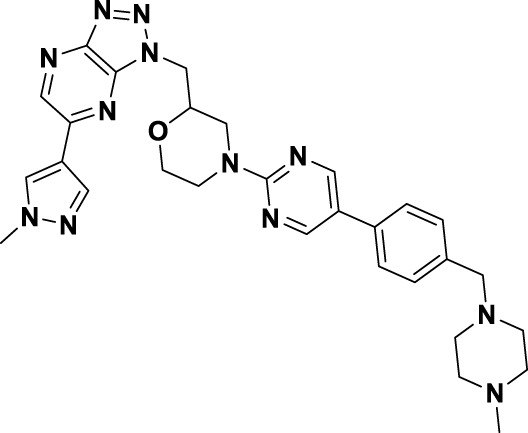
|
II | NCT05541822 | Recruiting | |
| I | NCT04052971 | Recruiting | |||
| I | NCT05248074 | Completed | |||
| Elzovantinib (8) |
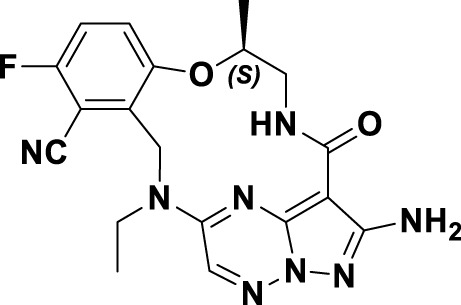
|
I/II | NCT03993873 | Active | |
| Glumetinib (9) |
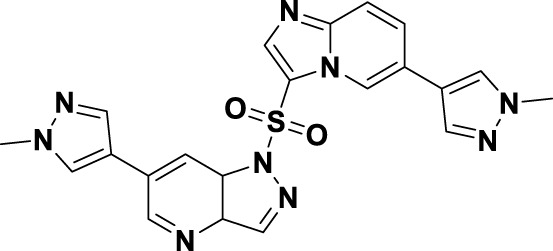
|
I/II | NCT04270591 | Unknown | |
| II | Cabozantinib (10) |
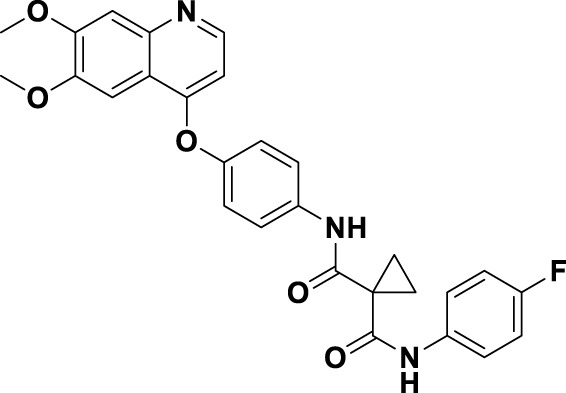
|
II | NCT02132598 | Terminated |
| I/II | NCT00596648 | Completed | |||
| I | NCT04173338 | Terminated | |||
| II |
NCT01639508 NCT05613413 |
Recruiting | |||
| II |
NCT00940225 NCT05200143 NCT03468985 NCT01866410 NCT02795156 |
Completed | |||
| III | NCT04471428 | Active | |||
| I/II | NCT04151563 | Withdrawn | |||
| II |
NCT04310007 NCT01708954 |
Active | |||
| Foretinib (11) |
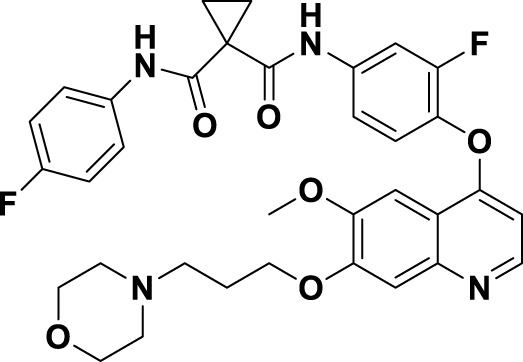
|
II | NCT02034097 | Withdrawn | |
| I/II | NCT01068587 | Completed | |||
| Glesatinib (12) |
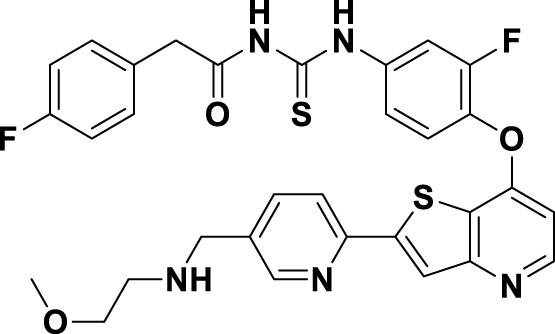
|
II | NCT02954991 | Terminated | |
| Merestinib (13) |
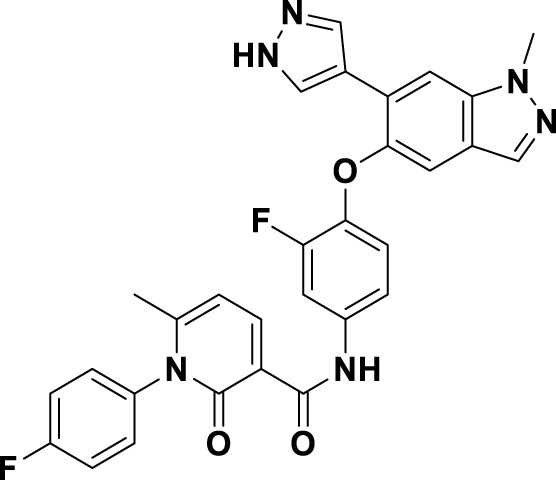
|
II | NCT02920996 | Terminated | |
| Ningetinib (14) |
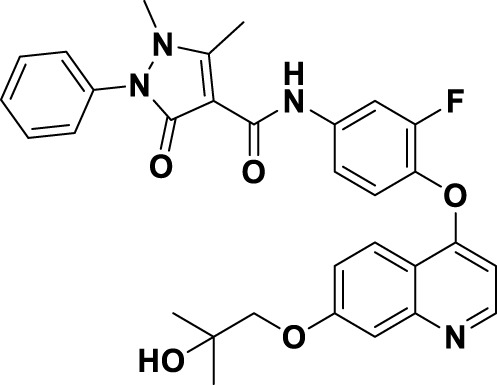
|
II | NCT03838848 | Terminated | |
| BMS-777607 (15) |
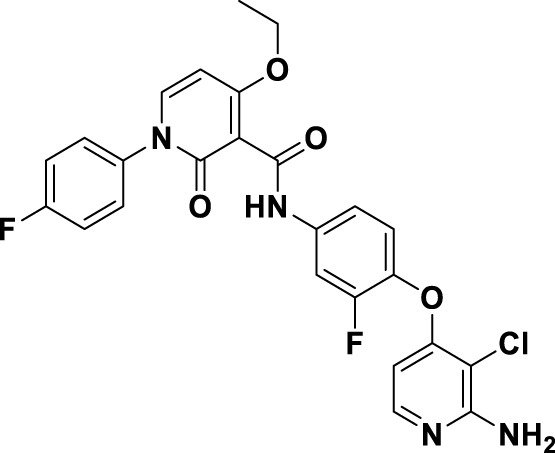
|
I/II | NCT00605618 | Completed | |
| I | NCT01721148 | Completed | |||
| III | Tivantinib (16) |
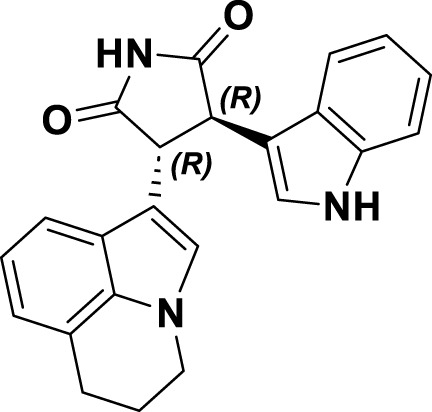
|
III | NCT01377376 | Terminated |
