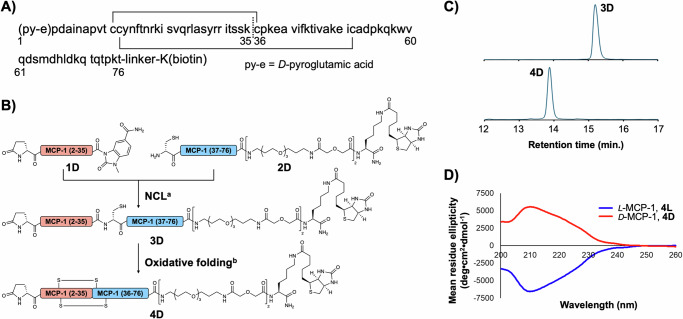
Fig. 1. Synthesis and evaluation of biotinylated D-MCP-1.
A Amino-acid sequence of MCP-1 with N-terminal pyroglutamic acid, pE, and C-terminal biotinylated lysine. Two disulfide bonds (solid lines) and a ligation site (a dashed line) are shown. B Synthetic scheme of biotinylated D-MCP-1 via NCL followed by air oxidation. Reaction conditions: (a) 200 mM MPAA, 50 mM TCEP, 6 M Gn·HCl, 0.2 M phosphate (pH 6.5), 37 °C; (b) AcOH/H2O, then NH3 aq. C Comparison of HPLC retention time between purified 3D and 4D. HPLC peaks were monitored at 220 nm in the linear gradient with water/acetonitrile containing 0.1% TFA. The gradient of HPLC: acetonitrile 20–40% for 20 min. D CD spectra of synthetic MCP-1. 4D and 4 L (10 µM) dissolved in PBS (pH7.4) were measured. Source data are provided as a Source Data file.