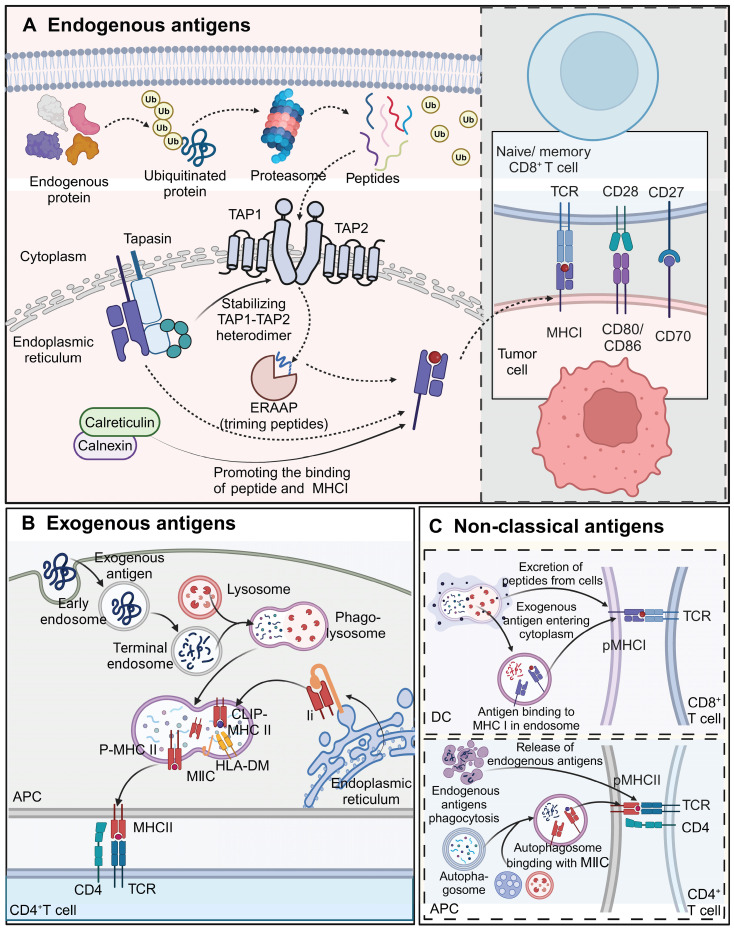
Figure 4.
Processing and presentation of different types of antigens. A) Endogenous proteins are ubiquitinated and degraded into peptides by the proteasome. Peptides are transported by the TAP1-TAP2 complex into the endoplasmic reticulum, where they are trimmed by ERAAP and loaded onto MHC I molecules. Calreticulin and calnexin assist in peptide loading and stabilization of MHC I. Once the peptide-MHC I complex is formed, it is transported to the cell surface to be recognized by CD8+ T cells, promoting cytotoxic T cell responses against tumor cells expressing these antigens. Co-stimulatory molecules such as CD80/CD86 and CD70 further enhance T cell activation. B) Exogenous antigens are taken up by APCs via endocytosis or phagocytosis. These antigens are processed in lysosomes and loaded onto MHC II molecules within the MIIC compartment, facilitated by HLA-DM, which removes CLIP from the MHC II complex. The peptide-MHC II complex is transported to the cell surface, where it is recognized by CD4+ T cells. This interaction triggers helper T cell activation and subsequent immune responses. C) Exogenous antigens can also enter the cytoplasm of APCs and be cross-presented on MHC I molecules, enabling CD8+ T cell activation. Additionally, endogenous antigens can be processed through autophagy, with autophagosomes delivering cytoplasmic contents, including endogenous antigens, to the MIIC for loading onto MHC II. Both cross-presentation and autophagy-mediated presentation allow for the activation of distinct subsets of T cells, broadening immune recognition of tumor antigens. The images in the figures were created using BioRender (https://www.biorender.com/).