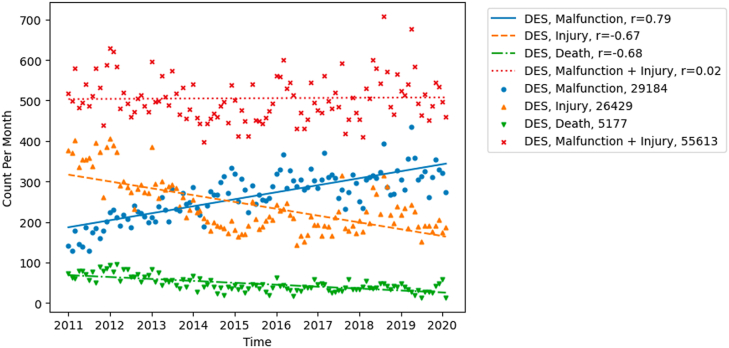
Fig. 2.
Trends in reported adverse events for DES in FDA MAUDE. The patient outcome Death, injury, and malfunction reports. Injury reports and malfunction reports compared to the summation of injury and malfunction reports. DES drug-eluting stents. FDA the U.S. Food and Drug Administration. MAUDE Manufacturer and User Facility Device Experience database.