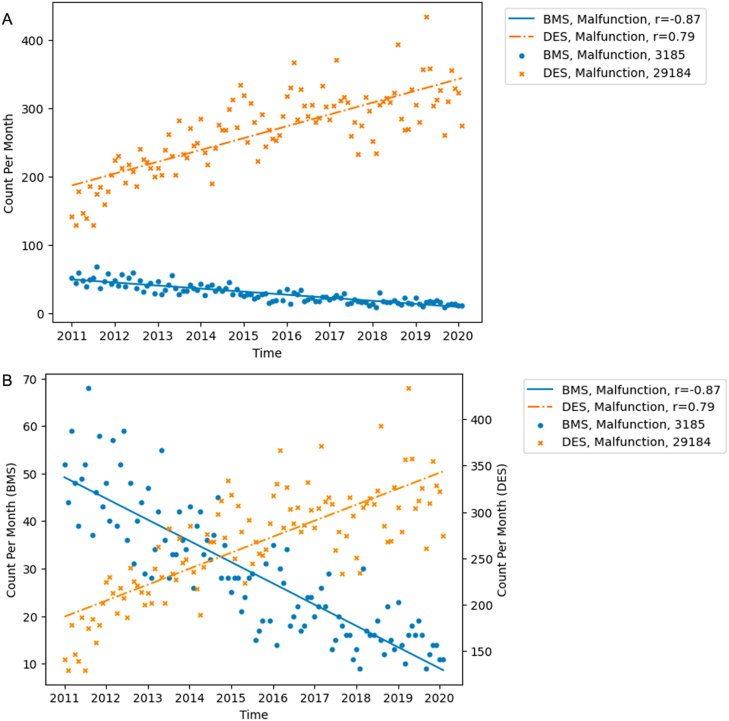
Fig. 3.
Comparison between DES and BMS for reported malfunctions in FDA MAUDE. (A) Single scale to show absolute trends. (B) Two scales to show relative trends. DES drug-eluting stents. BMS bare metal stents. FDA the U.S. Food and Drug Administration. MAUDE Manufacturer and User Facility Device Experience database.