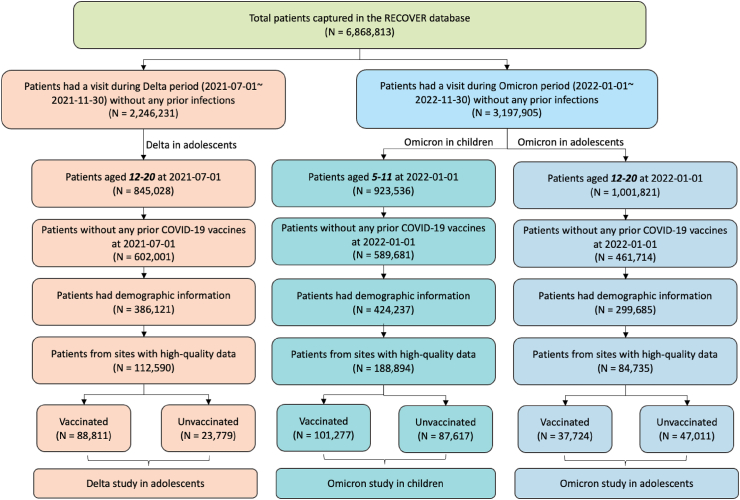
Fig. 1.
Selection of participants for the three study cohorts evaluating the overall effectiveness, direct and indirect effect of the BNT162b2 vaccine on long COVID outcomes in (1) adolescents aged 12–20 years during the period when the Delta variant was prevalent, (2) children aged 5–11 years and (3) adolescents aged 12–20 years during the period when the Omicron variant was prevalent.