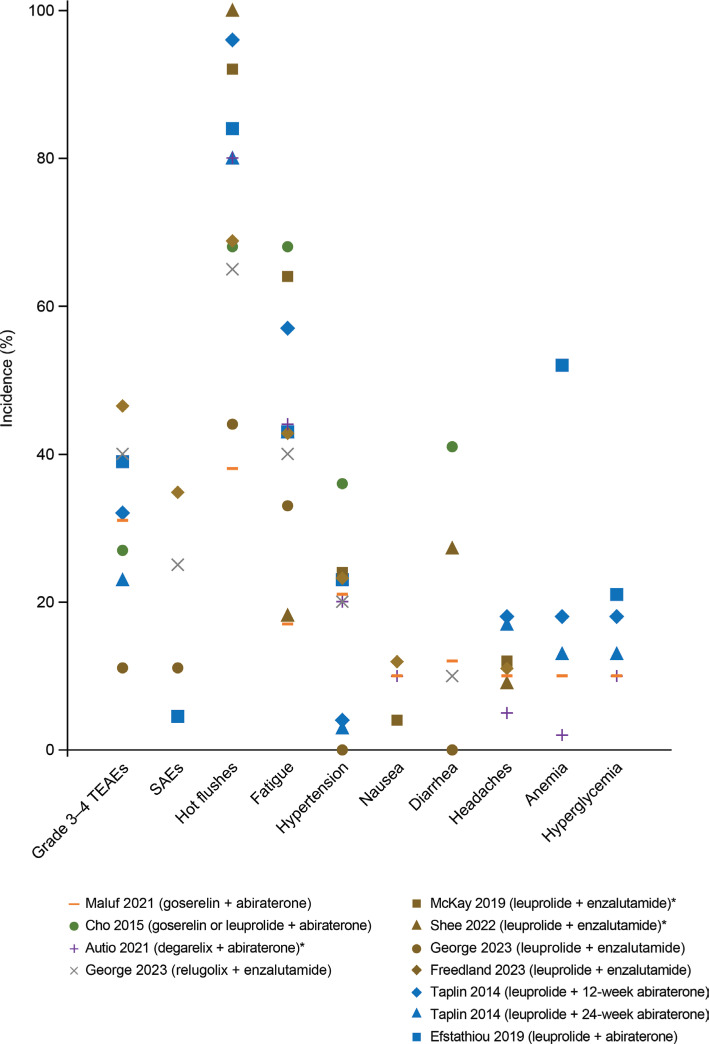
Figure 4.
Incidence of adverse events in combination therapy with LHRH-R agonists and antagonists with NHAs. Each dot represents the percentage of adverse events reported in a study; a dot at 0% indicates that the incidence was reported as 0% in the article. AST, ALT, and hypertension were defined as an adverse event of special interest in Taplin et al.97. *Study reported only the incidence of treatment-related adverse events. ALT, alanine aminotransferase; AST, aspartate aminotransferase; LHRH-R, luteinizing hormone-releasing hormone receptor; NHA, next-generation hormonal agent (i.e., abiraterone or enzalutamide). SAE, serious adverse events; TEAEs, treatment-emergent adverse events.