Abstract
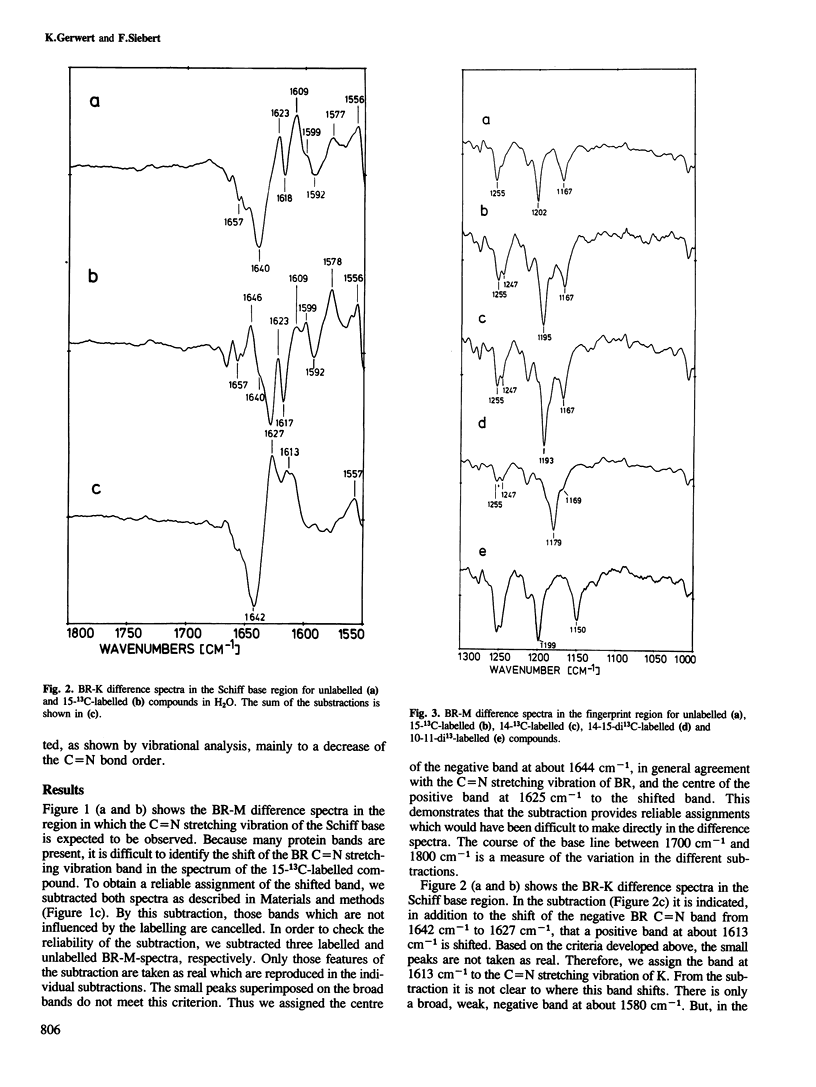
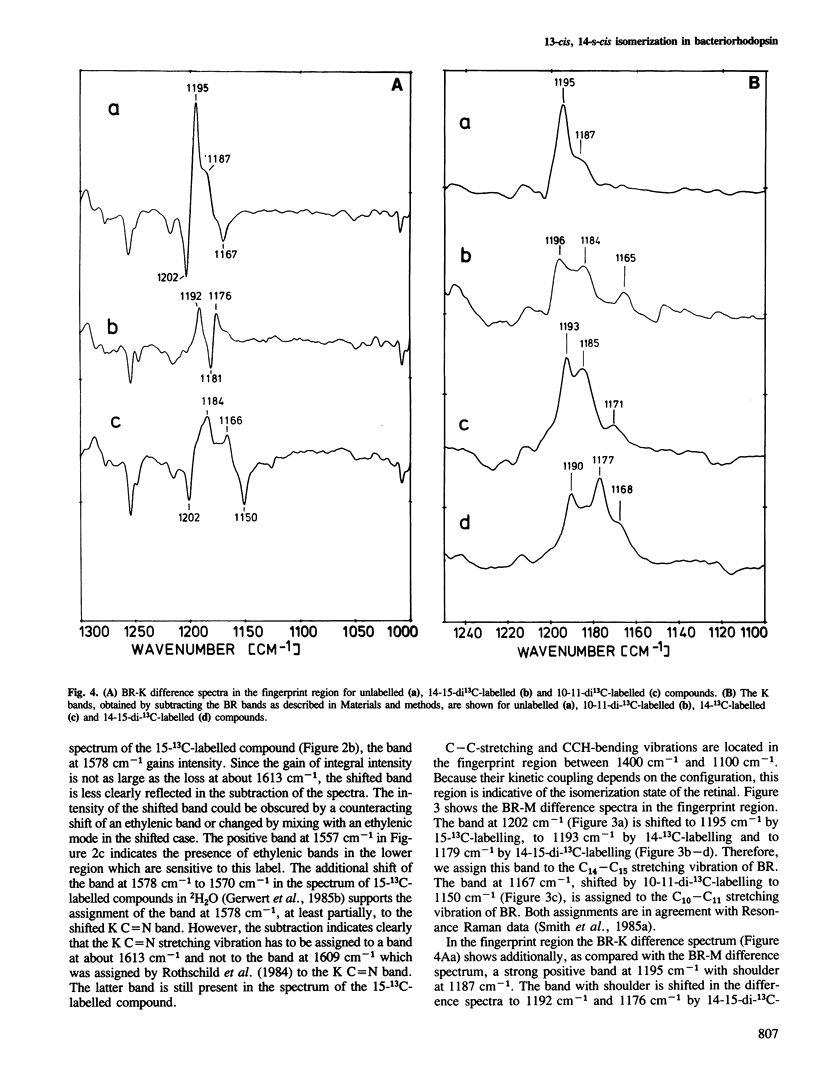
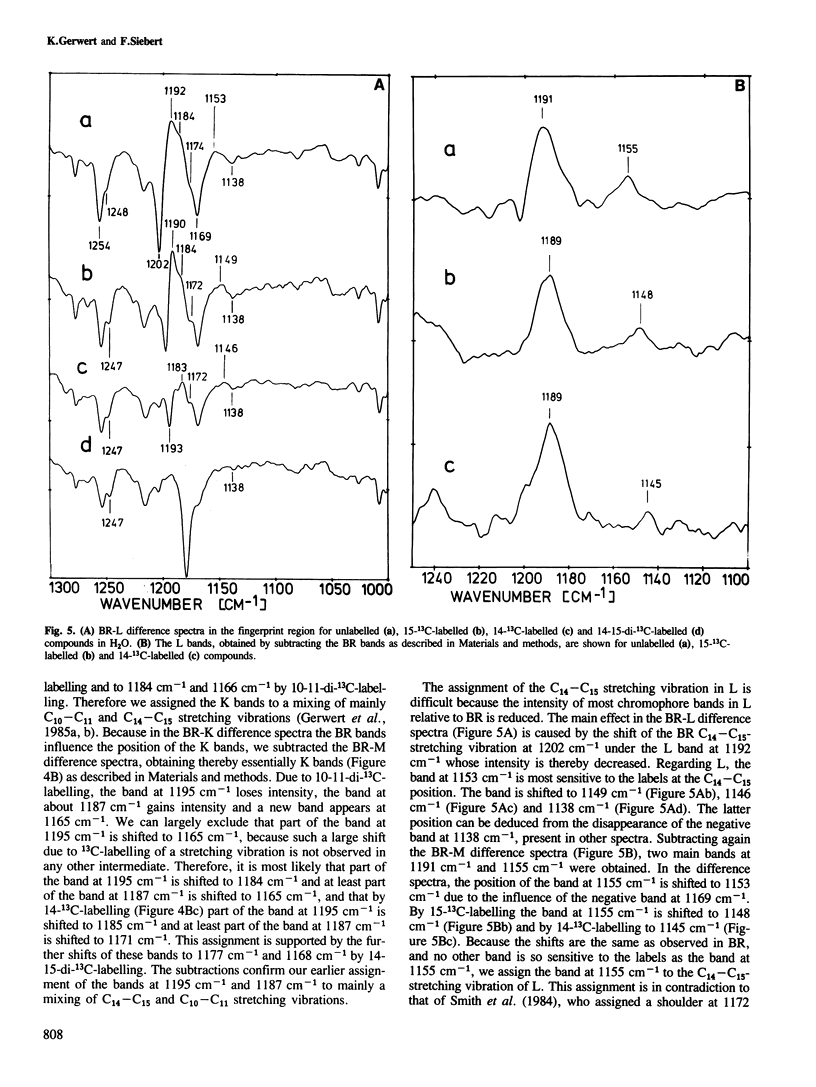
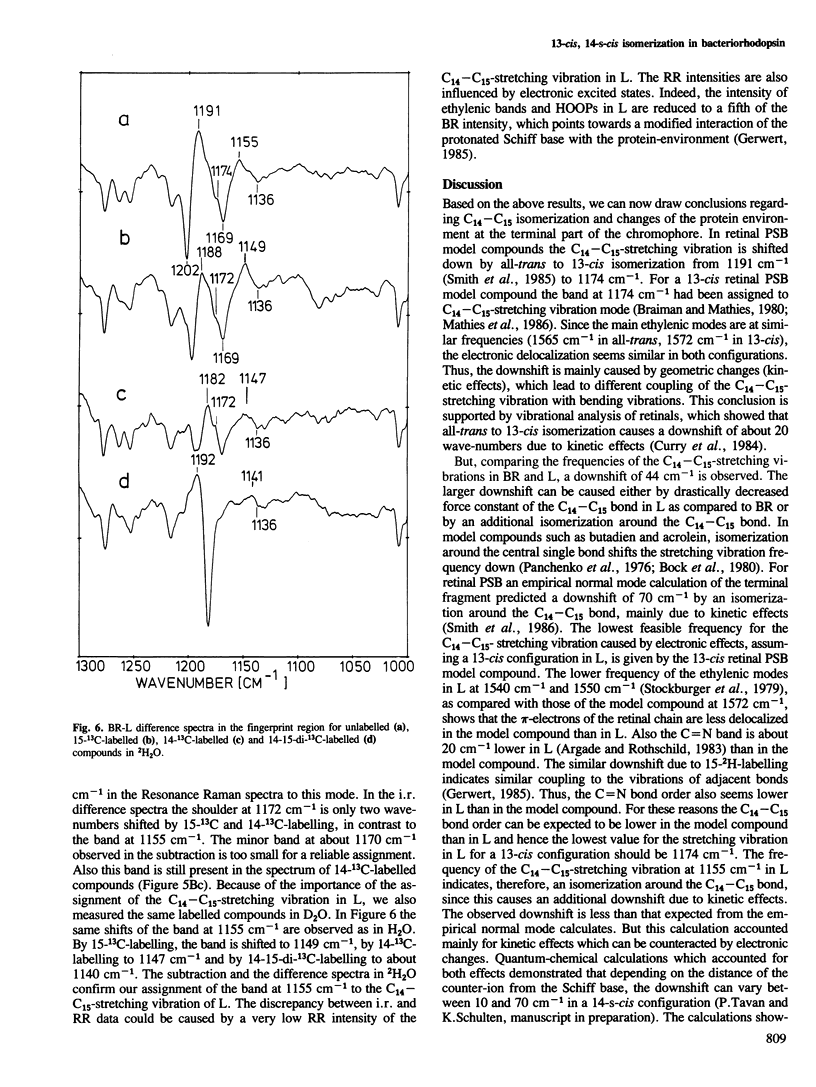
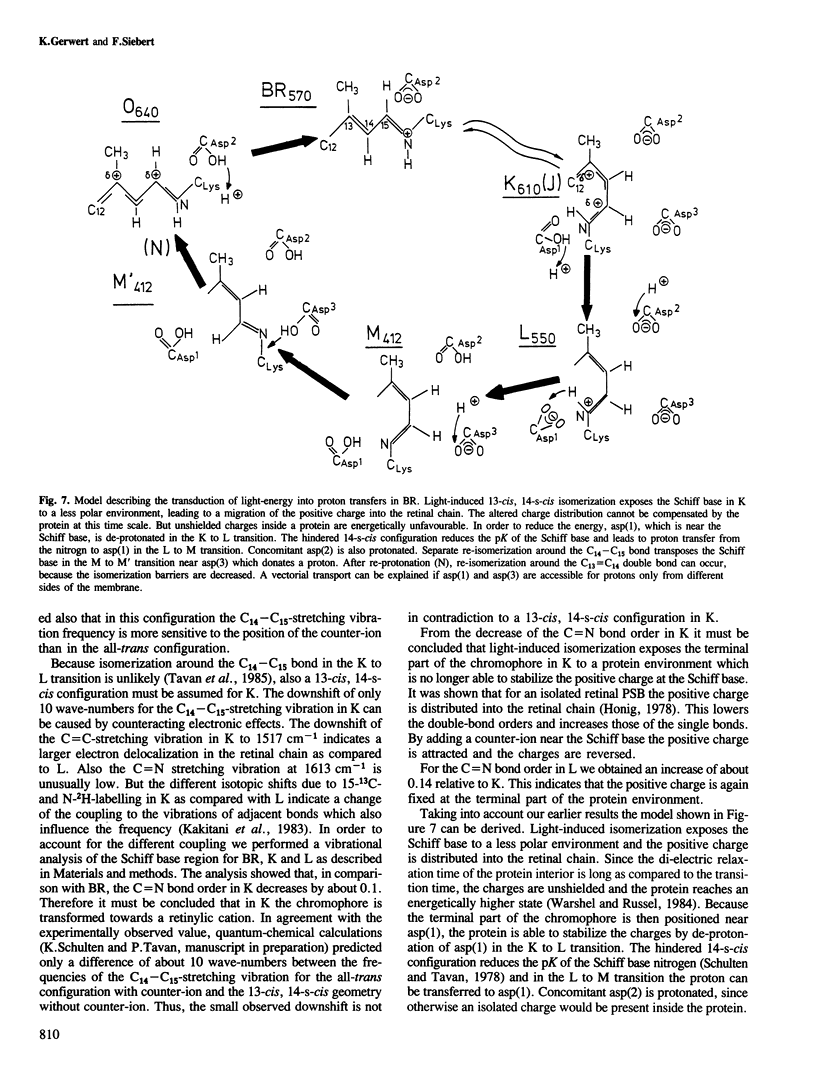
We have obtained by Fourier transformed infra-red (FTIR)-spectroscopy BR-K, BR-L and BR-M difference spectra of bacteriorhodopsin regenerated with isotopically labelled retinals. Thereby, we are able to assign reliably the C14–C15 and C=N stretching vibrations of the various intermediates. The lower C14–C15 stretching vibration frequency in L as compared with 13-cis protonated Schiff base model compounds indicates a 13-cis, 14-s-cis configuration of the retinal in this species. The unusually low C=N stretching vibration in K at 1615 cm−1 indicates less stabilization of the positive charge at the Schiff base by the protein environment. Based on these results, a mechanism is suggested by which the stored light energy is transformed into proton transfers.
Keywords: FTIR, bacteriorhodopsin, vibrational analysis, proton pump mechanism, retinal isotopic derivatives
Full text
PDF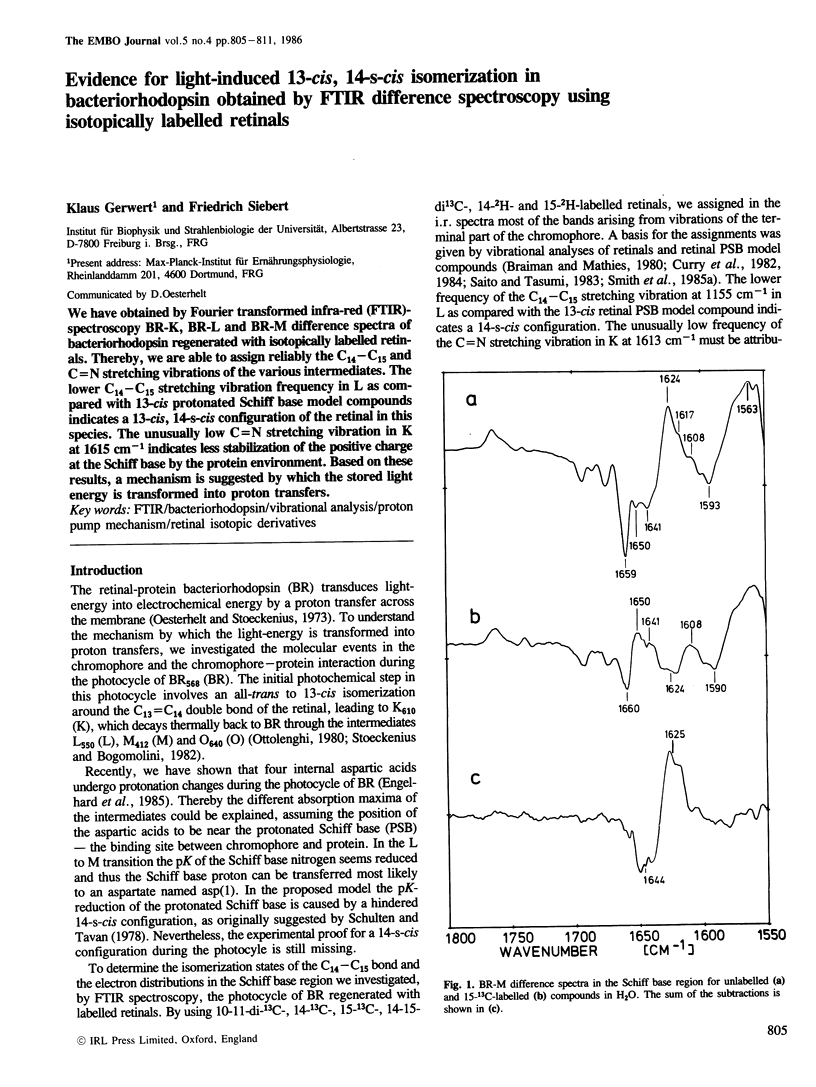

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braiman M., Mathies R. Resonance Raman evidence for an all-trans to 13-cis isomerization in the proton-pumping cycle of bacteriorhodopsin. Biochemistry. 1980 Nov 11;19(23):5421–5428. doi: 10.1021/bi00564a042. [DOI] [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Rothschild K. J., Roepe P., Lugtenburg J., Pardoen J. A. Fourier transform infrared evidence for Schiff base alteration in the first step of the bacteriorhodopsin photocycle. Biochemistry. 1984 Dec 4;23(25):6103–6109. doi: 10.1021/bi00320a031. [DOI] [PubMed] [Google Scholar]
- Schulten K., Tavan P. A mechanism for the light-driven proton pump of Halobacterium halobium. Nature. 1978 Mar 2;272(5648):85–86. doi: 10.1038/272085a0. [DOI] [PubMed] [Google Scholar]
- Siebert F., Mäntele W. Investigation of the primary photochemistry of bacteriorhodopsin by low-temperature Fourier-transform infrared spectroscopy. Eur J Biochem. 1983 Feb 15;130(3):565–573. doi: 10.1111/j.1432-1033.1983.tb07187.x. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Lugtenburg J., Mathies R. A. Determination of retinal chromophore structure in bacteriorhodopsin with resonance Raman spectroscopy. J Membr Biol. 1985;85(2):95–109. doi: 10.1007/BF01871263. [DOI] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Mathies R. A., Pardoen J. A., Winkel C., van den Berg E. M., Lugtenburg J. Vibrational analysis of the all-trans retinal protonated Schiff base. Biophys J. 1985 May;47(5):653–664. doi: 10.1016/S0006-3495(85)83961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. O., Myers A. B., Pardoen J. A., Winkel C., Mulder P. P., Lugtenburg J., Mathies R. Determination of retinal Schiff base configuration in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2055–2059. doi: 10.1073/pnas.81.7.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockburger M., Klusmann W., Gattermann H., Massig G., Peters R. Photochemical cycle of bacteriorhodopsin studied by resonance Raman spectroscopy. Biochemistry. 1979 Oct 30;18(22):4886–4900. doi: 10.1021/bi00589a017. [DOI] [PubMed] [Google Scholar]
- Tavan P., Schulten K., Oesterhelt D. The effect of protonation and electrical interactions on the stereochemistry of retinal schiff bases. Biophys J. 1985 Mar;47(3):415–430. doi: 10.1016/S0006-3495(85)83933-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga F., Ebrey T. The blue membrane: the 3-dehydroretinal-based artificial pigment of the purple membrane. Biochemistry. 1978 May 16;17(10):1915–1922. doi: 10.1021/bi00603a018. [DOI] [PubMed] [Google Scholar]
- Warshel A., Russell S. T. Calculations of electrostatic interactions in biological systems and in solutions. Q Rev Biophys. 1984 Aug;17(3):283–422. doi: 10.1017/s0033583500005333. [DOI] [PubMed] [Google Scholar]
- Watts T. H., Kay C. M., Paranchych W. Spectral properties of three quaternary arrangements of Pseudomonas pilin. Biochemistry. 1983 Jul 19;22(15):3640–3646. doi: 10.1021/bi00284a016. [DOI] [PubMed] [Google Scholar]


