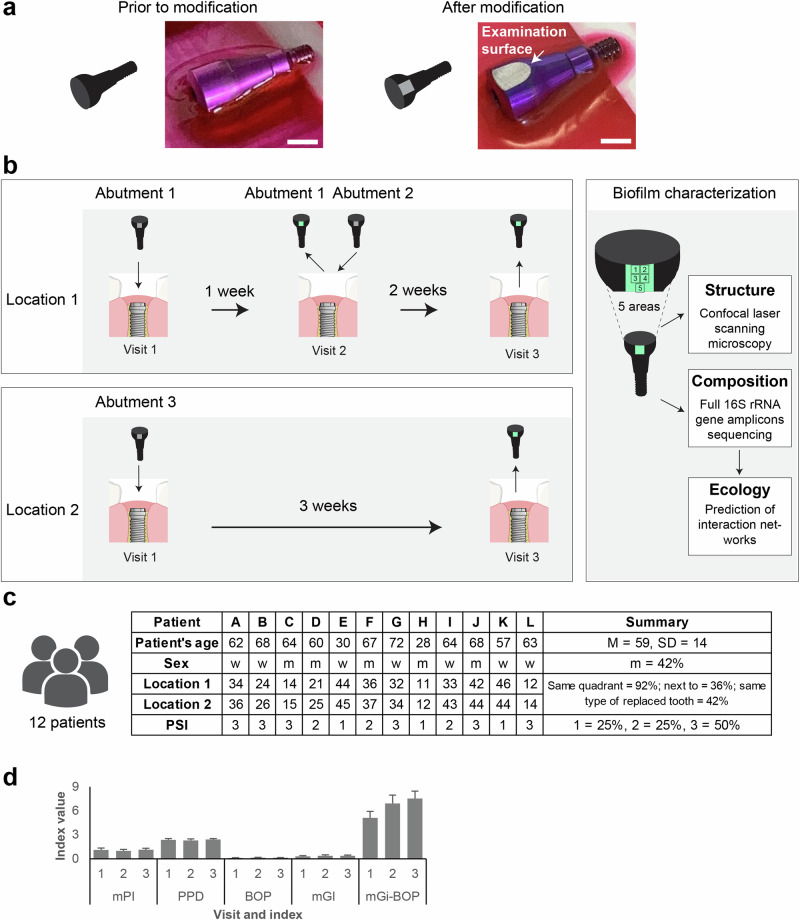
Fig. 1. Experimental setting for atraumatic biofilm investigation and clinical parameters.
a Modified temporary implant abutments with a flat examination surface were manufactured for each patient. Bar: 4 mm. b Abutments were inserted at two sites. Biofilm structures were analyzed using confocal microscopy (at five distinct areas), and the composition of the biofilm was characterized through 16S rRNA gene amplicon sequencing. c Demographic and clinical data of the participating patients. d Dynamics of clinical parameters: Modified Plaque Index (mPI), Probing Pocket Depth (PPD), Bleeding On Probing (BOP), modified Gingival Index (mGI) and Mucositis severity (mGi-BOP). Data refers specifically to implant abutment sites.