Abstract
A diarylurea-containing phosphine ligand-modulated stereoinvertive O-glycosylation with primal furanosyl and pyranosyl ortho-alkynylbenzoate (ABz) donors under gold(I) catalysis is disclosed. Both α- and β-configured glycosides could be obtained from the corresponding stereochemically pure β- and α-glycosyl donors with high yields and good to excellent stereoselectivities, respectively. This method accommodates a variety of glycosyl donors and alcoholic acceptors, leading to both 1,2-cis and 1,2-trans glycosidic linkages, and has been applied to the convenient preparation of a series of linear arabinan glycans. Mechanistic investigations reveal that the counteranion could bridge the diarylurea residue on the phosphine ligand with the alcoholic acceptor via hydrogen bond interactions, thereby permitting stereoinvertive displacement at the anomeric position.
Introduction
Glycans and glycoconjugates are ubiquitous in living organisms, participating in a wide array of biological processes and physiological responses such as cell signaling and immune response.1 Glycosidic bonds which connect monosaccharide subunits or aglycones to constitute complex carbohydrates hold diverse stereochemical attributes.2 For instance, the fungal glucan PS-I contains 1,2-trans β-glycosidic linkages, whereas glucan PS-II bears solely 1,2-cis α-glycosidic linkages;3 both 1,2-trans and 1,2-cis glycosidic bonds are present in the mycobacterial cell-wall arabinan constituent (Scheme 1A).4 Therefore, glycosylation reactions with precise and predictable control of both anomeric configurations are in demand.5
Scheme 1. Brief Introduction of Stereoselective Glycosylation.
Due to the diverse electronic and steric properties of the coupling partners as well as the varying reaction parameters, a glycosylation reaction occurs following a fluctuating mechanism within the SN1–SN2 boundary, rendering the stereocontrol of this transformation highly challenging (Scheme 1B).6 Toward this issue, substantial efforts have been made to achieve stereoselective glycosylation (Scheme 1C).7 Classic strategies include the anchimeric assistance of participating groups,8 directed aglycone delivery through fragile covalent bonds or hydrogen-bonding interactions,9 modulation of solvents and exogenous nucleophiles,10 and control of the conformation of glycosyl oxycarbenium species using rigid protection patterns.11 Recently, catalytic approaches involving chiral promoters such as phosphoric acids and bis-thioureas,12 cooperative acid–base catalysis,13 organoboron-mediated aglycone delivery,14 nucleophilic activation of glycosyl halides with phenanthroline,15 palladium-promoted anomeric replacement of thioglycosides,16 etc.,17 have been developed. However, these strategies either require special stereodirecting groups on the coupling partners or accommodate limited types of sugar donors, thus calling for more facile and general stereocontrol methods.18
Taking advantage of the alkynophilicity of cationic gold(I) complexes,19 our group disclosed a highly efficient and extremely mild gold(I)-catalyzed glycosylation protocol with bench-stable glycosyl ortho-alkynylbenzoate (ABz) donors in 2008, which has gained broad applications to the synthesis of complex carbohydrates.20,21 We later found that highly stereoselective formation of β-manno- and β-rhamnopyranosides could be achieved through the gold(I)-promoted one-pot anomerization/substitution sequence.22 Reviewing the established mechanism of the gold(I)-catalyzed glycosylation (Scheme 2A),23 we identified several challenges for developing a generally stereoselective version: (1) the glycosidic bond might be formed through either an SN1-like or SN2-like pathway, in which the dissociated glycosyl oxocarbenium C and the contact glycosyloxypyrylium B act as the stereodetermining species, respectively; (2) recombination of the isochromen-4-yl-gold(I) complex D with the glycosyl oxocarbenium C would cause the epimerization of donor 1; (3) the counteranion X– could affect the stereochemical outcome by generating a contact ion pair with glycosyl oxycarbenium C.
Scheme 2. Our Stereoselective Glycosylation Protocol.
Indeed, several stereoselective versions of the gold(I)-catalyzed glycosylation reactions with ABz donors have been realized. Wan and Zeng and co-workers utilized the hydrogen-bonding between the C3-axial NH group and exogenous triarylphosphine oxide to stabilize the proposed α-oxyphosphonium intermediate and thus enabled β-selective glycosylation of 3-amino sugars.24 The Zhang group installed a stereodirecting group onto the leaving group to achieve stereodivergent glycosylation via SN2 replacement (Scheme 1C).25 Recently, Li and colleagues exploited the remote participation of phosphine oxide to accomplish stereoselective synthesis of both α- and β-2′-deoxynucleosides.26 Nevertheless, these elegant tactics demand stoichiometric stereodirecting groups installed on either the sugar rings or the leaving group. We envisioned that a bifunctional gold(I) catalyst bearing a stereodirecting group could not only exhibit stereocontrol capability but also minimize the prefabrication of glycosyl donors. Although it has been documented that integrating a hydrogen-bond-donating functionality into gold(I) catalysts could improve the stereoinductive effect as well as catalytic efficiency,27 the implementation of this concept to gold(I)-catalyzed glycosylation with ABz donors remained underexplored. As such, we devised a series of diarylurea-containing phosphine-coordinated cationic gold(I) catalysts, which were expected to guide the nucleophilic attack of the glycosyl acceptor via hydrogen-bonding interactions and thus facilitate SN2-like glycosylation (Scheme 2B). Herein, we report the highly stereoselective glycosylation with primal ABz donors under the action of this type of bifunctional gold(I) catalysts. Preliminary mechanistic studies indicated that the multiple hydrogen bondings between the diarylurea motif, the counteranion, and the alcoholic acceptor were crucial to the stereocontrolled glycosylation.
Results and Discussion
Following the initial design, we started with the model coupling of anomerically pure perbenzyl d-xylofuranosyl ABz donor α-1a with an equimolar quantity of achiral 1-adamantanol (2a) under the promotion of 5 mol % bifunctional gold(I) catalyst (Table 1). The gold(I) catalyst was prepared prior to use by mixing the parental chloro(phosphine)gold(I) complex with a silver salt to avoid decomposition of the active cationic gold(I) species. We first prepared L1AuNTf2 and screened the reaction parameters (see SI, optimization section). To our delight, α-1a was nearly completely converted under the catalysis of L1AuNTf2 in binary PhCF3 and cyclohexane solvents at −25 °C, leading to 3a in a good 89% yield with a 1:14.8 α/β ratio (Table 1, entry 1). Notably, the use of drierite as a drying agent was critical to mitigate the unproductive hydrolysis of donor α-1a and therefore secured the high yield of coupled glycoside 3a. Next, we examined the substituents on the phosphine atom and the distal nitrogen atom (Table 1, entries 1–5). Changing the phenyl group on the phosphine atom to a bulky 1-adamantyl group resulted in improved stereoselectivities and comparable yields (L2 vs L1, L4 vs L3). Markedly, employment of L2AuNTf2 as the promoter gave rise to 3a in a high 87% yield with an outstanding α/β ratio of 1:19.3. Replacing the 3,5-bis(trifluoromethyl)phenyl group on the distal nitrogen atom with a less electron-withdrawing para-tolyl group decreased the stereoselectivity, possibly due to the attenuated hydrogen bond donating capacity of the urea moiety (L3 vs L1, L4 vs L2). Switching the spacer linking the phosphine and urea functionalities from a biphenyl group to shorter phenyl group caused inferior stereoselectivities, whether the urea motif was positioned ortho, meta, or para to the phosphine group (L5–L7 vs L1). The conventionally used Ph3PAuNTf2 exerted only a modest β-selectivity (α/β = 1:6.3), verifying the stereoinducing effect of the appended diarylurea function on the ligand.
Table 1. Condition Optimizationa.
| Entry | Reaction partners | L | X | Conv of 1a (%) | Yield (%) of 3 (α:β) |
|---|---|---|---|---|---|
| 1 | α-1a and 2a | L1 | NTf2 | 99 | 89 (1:14.8) |
| 2 | L2 | NTf2 | 99 | 87 (1:19.3) | |
| 3 | L2 | OTf | 99 | 98 (1:1.6) | |
| 4 | L3 | NTf2 | 99 | 95 (1:10.2) | |
| 5 | L4 | NTf2 | 99 | 86 (1:12.8) | |
| 6 | L5 | NTf2 | 99 | 91 (1:13.2) | |
| 7 | L6 | NTf2 | 99 | 91 (1:11.7) | |
| 8 | L7 | NTf2 | 99 | 99 (1:9.9) | |
| 9 | PPh3 | NTf2 | 99 | 94 (1:6.3) | |
| 10 | β-1a and 2a | L2 | NTf2 | 99 | 94 (4.2:1) |
| 11 | β-1a and 2b | L2 | NTf2 | 99 | 97 (7.4:1) |
| 12 | L2 | OMs | <5 | N. D. | |
| 13 | L2 | OTs | <5 | N. D. | |
| 14 | L2 | BF4 | 38 | 35 (>20:1) | |
| 15 | L2 | PF6 | 99 | 84 (13.1:1) | |
| 16 | L2 | OTf | 99 | 98 (15.4:1) | |
| 17 | PPh3 | OTf | 99 | 96 (1:2.2) | |
| 18 | L8 | OTf | 99 | 98 (1:1.8) | |
| 19 | L2 | BArF4 | 99 | 99 (1.7:1) | |
| 20 | PPh3 | BArF4 | 86 | 82 (1.8:1) |
Reactions were carried out at 0.05 mmol scale. Yields and conversions (conv) were determined by 1H NMR analysis using 1,3,5-trimethoxybenzene as the internal standard. The ratios of 3a epimers were collected by the 1H NMR analysis of the crude reaction mixture. N. D. = not determined. Ad = 1-adamantyl. BArF4 = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate.
Having identified the optimal conditions for stereoinvertive β-glycosylation (Table 1, entry 2), we moved forward to explore the complementary α-glycosylation with the corresponding ABz donor β-1a. The condensation of β-1a with 1-adamantanol (2a) and d-glucose-derived primary alcohol 2b under the promotion of bifunctional L2AuNTf2 delivered the coupled glycosides 3a and 3b with moderate 4.2:1 and 7.4:1 α/β ratios, respectively (entries 10 and 11). In order to adequately reflect the stereodirecting potential of the bifunctional gold(I) catalysts, 2b was chosen as the glycosyl acceptor in the following optimizations. We envisaged that the counteranion played a pivotal role in both coupling efficiency and stereocontrol because it could not only coordinate to the cationic gold center but also interact with the hydrogen-bond-donating urea motif as well as the glycosyl oxycarbenium species.6,28 Therefore, we tested various counteranions including bistriflimidate (Tf2N–), mesylate (MsO–), tosylate (TsO–), tetrafluoroborate (BF4–), hexafluorophosphate (PF6–), and triflate (TfO–) (Table 1, entries 11–16). Donor β-1a was hardly activated when MsO– and TsO– were invoked as counteranions, presumably because of their relatively high affinity to the cationic gold(I) center, underscoring the importance of the balance between the coordinating ability and hydrogen-bonding basicity of the counteranion.28 Use of L2AuBF4 as the catalyst led only to a sluggish 38% conversion despite the exclusive α-selectivity. Interestingly, utilization of weakly coordinating TfO– as the counteranion resulted in nearly quantitatively generation of coupled 3b with unexpectedly high stereoinversion (α/β = 15.4:1). We conjectured that TfO– was possibly locked to the diarylurea group by a hydrogen-bonding interaction, therefore impeding the unwanted formation of β-glycoside from the corresponding α-glycosyl triflate. Control experiments with Ph3PAuOTf and L8AuOTf as the catalysts provided 3b in 96% and 98% yields with reversed 1:2.2 and 1:1.8 α/β ratios, respectively, once again demonstrating the stereoinducing capacity of the diarylurea group embedded in the phosphine ligand backbone. Moreover, the stereoinducing effect of the embedded urea function almost vanished when BArF4– was exploited instead of TfO–, implying the importance of the hydrogen-bonding basicity of the counteranion (entries 16 vs 19).
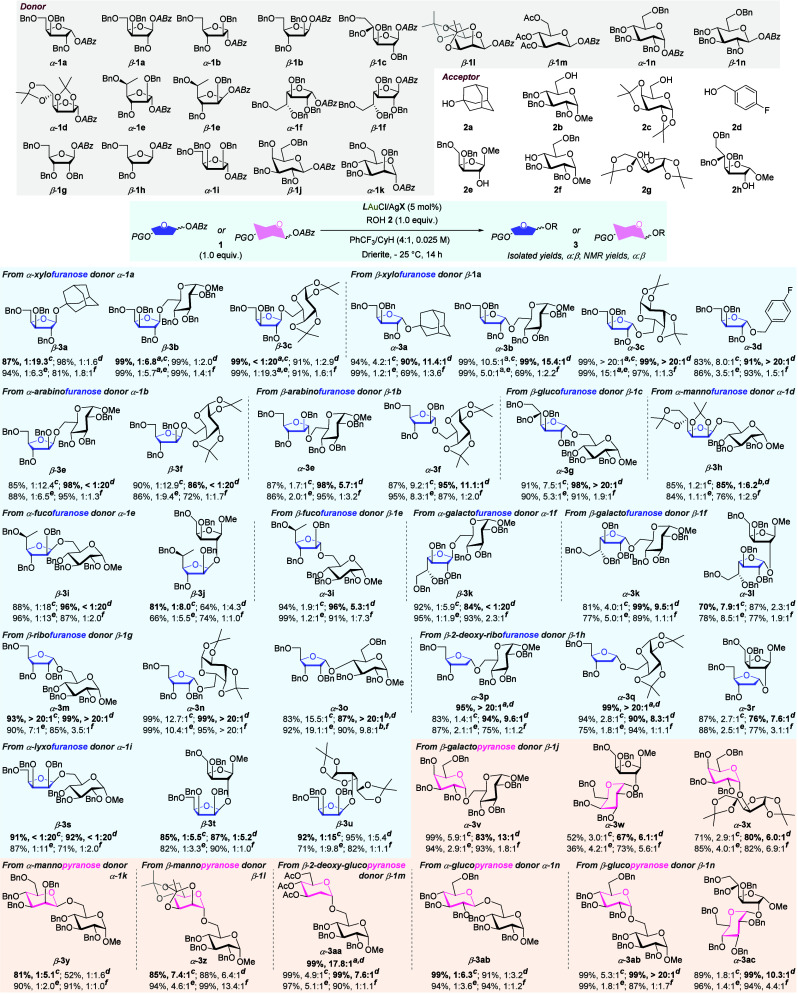
With the optimized gold(I)-catalyzed stereoinvertive glycosylation conditions in hand, we turned to explore the substrate scope (Scheme 3). Thus, a diverse set of 19 ABz donors and 8 acceptors were prepared to assess the stereoinducing performance of this bifunctional phosphine ligand enabled SN2-like glycosylation. Notably, each coupling reaction was carried out four times under the promotion of L2AuNTf2, L2AuOTf, Ph3PAuNTf2, and Ph3PAuOTf, respectively. Gratifyingly, activation of the pentofuranosyl ABz donors derived from d-xylose (1a), d-arabinose (1b), d-ribofuranose (1g), 2-deoxy-d-ribofuranose (1h), and d-lyxofuranose (1i) with bifunctional gold(I) catalysts delivered the corresponding furanosides with 83–99% yields and good to excellent stereoselectivities (3a–3f, 3m–3u). Compared to the conventional phosphine ligand Ph3P, the use of diarylurea-containing L2 secured a high degree of configurational inversion at the anomeric center for both α- and β-donors, especially when the counteranion was TfO–. Notably, increasing the loading of acceptor to 2.0 equiv significantly amplified the α-selectivity of glycosylation with 2-deoxy-d-ribofuranosyl donor β-1h, affording α-3p and α-3q with nearly quantitative yields and >20:1 α/β ratios. We proposed that the overstoichiometric acceptor expedited the desired SN2-like process by sufficient interplay with the stereoinducing biarylurea group. Hexofuranosyl ABz donors derived from d-glucose (1c), d-mannose (1d), l-fucose (1e), and d-galactose (1f) were also competent to undergo the stereoinvertive glycosylation, leading to the coupled glycosides with 5.3:1 to >20:1 stereopreferences (3g–3l). The need for an additional portion of gold(I) catalyst and the slightly lower stereoselectivity of β-3h (85%, α/β = 1:6.2) could be ascribed to the steric shielding effect of the cis-fused acetonide at the C2/C3 positions of donor α-1d. The relatively low yields of α-3l and α-3w were due to the incomplete conversion of glycosyl donors. In addition, condensation of ABz donor β-1g with p-tolyl 6-O-tert-butyldiphenylsilyl-1-thio-d-mannopyranoside has also been conducted, giving the corresponding 2-O-, 3-O-, and 4-O-glycosylated products in 8.0%, 35%, and 6.5% yields, respectively (for details, see SI). Notably, all three regioisomers were obtained with exclusive α-selectivities, indicating the potential of the present protocol in the glycosylation with minimally protected acceptors.
Scheme 3. Substrate Scope.
Reactions were carried out at 0.05 mmol scale, using equimolar quantities of donor and acceptor.
Using 2 equiv of ROH.
Adding 10% loading of cat. in two portions.
L2AuCl/AgNTf2.
L2AuCl/AgOTf.
PPh3AuCl/AgNTf2.
PPh3AuCl/AgOTf.
The ligand-enabled stereoinvertive glycosylation reactions with pyranosyl ABz donors 1j–1n were also tested. As expected, the pyranosylation reactions occurred smoothly under the catalysis of the bifunctional gold(I) catalysts, offering the desired disaccharides 3v to 3ac with moderate to high stereopreferences. Using L2AuOTf as the promoter instead of Ph3PAuOTf drastically improved the α/β ratio of galactopyranoside 3v from 1.8:1 to 13:1. In contrast, the stereoinducing character of the bifunctional phosphine ligand almost vanished in the synthesis of congested α-galactopyranosides 3w and 3x. We speculated that the interplays of bulky acceptors 2e and 2g with the diarylurea motif were interrupted by steric hindrance. Similar to the results of 2-deoxyfuranosides 3p and 3q, 2-deoxy-d-glucopyranoside 3aa was obtained with a high α/β ratio of 17.8:1 by using 2.0 equiv of acceptor 2b. Taken together, diarylurea-containing bifunctional gold(I) catalysts L2AuNTf2 and L2AuOTf were proven to be able to promote highly stereoselective SN2-like glycosylation with primal ABz donors. It is noted that the performance of L2AuNTf2 and L2AuOTf varied across the tested coupling pairs, and thus both of them should be assessed to identify the better one for a given glycosylation.
To examine the synthetic potential of the present stereoinvertive glycosylation, we performed an iterative glycosylation sequence for the synthesis of linear arabinan oligosaccharides 4–6, some of which were related to the cell envelope of Mycobacteria (Scheme 4).4 Key synthons were recursive d-arabinofuranosyl ABz donors α-1o and β-1o in which C5-OH could be selectively liberated after each glycosylation. The coupling of β-1o and α-1o with methyl d-arabinofuranoside α-2i under the catalysis of L2AuOTf took place well, providing the coupled glycosides α-3ad and β-3ad in 98% and 97% yields with 8.9:1 and 1:19 α/β ratios, respectively. A typical round of sugar chain growth included two steps: (1) removal of the acetyl group with sodium methoxide and (2) the stereoinvertive glycosylation promoted by L2AuOTf with either α-1o or β-1o. Thus, tetrasaccharide 5a and pentasaccharide 6b were constructed after two and three rounds of sugar chain growth, respectively, with 71% to 91% yields and 1:6.8 to 18:1 stereopreferences in the glycosylation steps. By the same token, pentasaccharide 6c was stereoselectively synthesized from ABz donor α-1o and methyl d-arabinofuranoside β-2i.
Scheme 4. Iterative Glycosylation for the Syntheses of Arabinan Oligosaccharides.

At this stage, efforts were made to probe the origin of stereocontrol in the present glycosylation protocol. Replacement of the urea group in L2 with carbonate and carbamate groups led to analogous phosphine ligands L9–L11. Compared to Ph3P, the newly prepared L9–L11 displayed only a marginal stereoinducing effect in the gold(I)-promoted synthesis of 3b, although with high yields (Scheme 5). Adding 5 mol % hydrogen bond donor 7a made almost no difference to the results of the Ph3PAuOTf-catalyzed glycosylation of β-1a with 2b. These parallel experiments underscored the indispensable role of the covalently appended diarylurea functionality in the stereocontrol. The single-crystal structure of the gold(I) complex containing the bifunctional phosphine ligand could help us to understand the role of the embedded urea motif, although the interactions observed in the solid state might not occur in solution. The X-ray structure of chloride-bridged bisgold(I) complex [Cl(L2Au)2]OTf revealed the interaction of triflate anions with the NH groups of urea, suggesting that the counteranion could bind to the urea motif of the bifunctional gold(I) catalysts, and the resulting adducts guided the nucleophilic attack of the acceptor (CCDC 2373130; for details, see SI).27b,29
Scheme 5. Comparative Experiments on the H-Bonding Donor Functionalities.
Adding 5% loading of 7a.
The inversion of the anomeric configuration indicated that the departure of the leaving group was probably synchronous with the formation of a new glycosidic bond, making activated glycosyloxypyrylium B pivotal to the stereochemical outcome. Since the gold(I) center of glycosyloxypyrylium B has been coordinatively saturated, tetrabutylammonium was used to balance the negative charge of triflate and bistriflimide anions. Diarylurea 7a was invoked to act as the surrogate of the appended urea motif in bifunctional phosphine ligand L2. Thus, we set up an array of combinations of tetrabutylammonium salts 8a and 8b, diarylurea 7a, and alcohols 2b and 2d, to simulate the behaviors of the key species in the glycosylation reaction (Scheme 6).30 Mixing triflate salt 8a with an equimolar quantity of diarylurea 7a caused a significant downfield shift of the signals of urea NH proton signals (H4 and H5) and upfield shift of the signal of tetrabutylammonium α-protons (H1), by 3.20 ppm and −0.54 ppm, respectively. This fact supported a strong hydrogen-bonding interaction between the diarylurea motif and the triflate anion.30a Subjection of alcohol 2b to the mixture of triflate salt 8a and 7a altered the 1H NMR spectrum considerably, with the signals of urea NH protons (H4 and H5) and tetrabutylammonium α-protons (H1) shifted by 0.27 and 0.07 ppm, respectively. This phenomenon implied that the adduct resulting from the strong hydrogen bonding between TfO– and the urea function was able to show an appreciable directing effect on alcoholic acceptors. 1H NMR measurements using bistriflimide salt 8b have been performed. Notable deviations of the chemical shifts of characteristic protons were observed (0.36 ppm for H3, −0.21 ppm for H2) when bistriflimide salt 8b was combined with an equimolar amount of diarylurea 7a. It was noted that we failed to assign the signals of urea NH protons (H4 and H5) because they are severely overlapped with the signals of the aromatic protons. Addition of alcohol 2b impacted scarcely on the 1H NMR spectrum of the mixture of bistriflimide salt 8b and 7a, indicating a relatively weak directing capacity of the adduct of Tf2N– and the urea motif. The influence of the urea-triflate adduct on the 1H NMR spectra of simple alcohol 2d has also been estimated. As expected, a small but noticeable 0.04 ppm change of the chemical shift of benzylic protons H6 was witnessed when alcohol 2d, triflate salt 8a, and urea 7a were mixed. Based on these observations, we proposed that the ligand-enabled stereoinvertive glycosylation happened via an approximately intramolecular aglycone delivery pathway in which the glycosyl acceptor and the stereoinducing urea group were connected by the counteranion through hydrogen bondings, although the details of this multicomponent interaction remain unclear.
Scheme 6. NMR Experiments.
NMR measurements were performed using toluene-d8 (0.5 mL) as the deuterated solvent and equimolar quantity of each component at the 0.01 mmol scale.
Conclusion
In conclusion, we have demonstrated a facile and versatile protocol for gold(I)-catalyzed stereoinvertive glycosylation with primal ABz donors. This method features a urea-containing phosphine ligand, which enabled highly stereoselective synthesis of both α- and β-glycosides from the corresponding anomerically pure β- and α-configured ABz donors, respectively. A variety of furanosides and pyranosides bearing 1,2-cis, 1,2-trans, and 2-deoxy glycosidic linkages were successfully prepared with high yields and stereoselectivities. Mechanistic investigations supported a counteranion-bridged aglycone delivery process through hydrogen-bonding interactions. The highly stereoselective glycosylation reaction described herein does not require a specialized stereodirecting group on the glycosyl donor and will thus find broad applications to the construction of diverse carbohydrate molecules. In addition, the present highly stereoinvertive glycosylation method will also spur the development of stereoselective construction of the needed anomerically pure glycosyl esters.31
Acknowledgments
Financial support from the National Key Research & Development Program of China (2022YFA1304700, 2023YFA1508800), the National Natural Science Foundation of China (22031011, 22377140), the Key Research Program of Frontier Sciences of CAS (ZDBS-LY-SLH030), and the Shanghai Municipal Science and Technology Major Project is acknowledged. W.Z. thanks the funding support from the China Postdoctoral Science Foundation under Grant Number 2023M743666 and Shanghai Postdoctoral Excellence Program (No. 2023728).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c10698.
General experimental procedure, characterization of organic compounds, mechanistic experiments, NMR spectra, X-ray crystallographic data, and supplementary references (PDF)
Author Contributions
§ W.Z. and R.W. contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Varki A.; Cummings R. D.; Esko J. D.; Stanley P.; Hart G. W.; Aebi M.; Mohnen D.; Kinoshita T.; Packer N. H.; Prestegard J. H., et al. Essentials of Glycobiology, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 2022. [PubMed] [Google Scholar]
- Chen Y.; Liu Y.; Chen N.; Jin Y.; Yang R.; Yao H.; Kong D.-X. A chemoinformatic analysis on natural glycosides with respect to biological origin and structural class. Nat. Prod. Rep. 2023, 40, 1464–1478. 10.1039/D2NP00089J. [DOI] [PubMed] [Google Scholar]
- a Synytsya A.; Novák M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. 10.1016/j.carbpol.2012.09.077. [DOI] [PubMed] [Google Scholar]; b Liu J.-J.; Hou Y.-K.; Wang X.; Zhou X.-T.; Yin J.-Y.; Nie S.-P. Recent advances in the biosynthesis of fungal glucan structural diversity. Carbohydr. Polym. 2024, 329, 121782. 10.1016/j.carbpol.2024.121782. [DOI] [PubMed] [Google Scholar]
- a Holzheimer M.; Buter J.; Minnaard A. J. Chemical synthesis of cell wall constituents of Mycobacterium tuberculosis. Chem. Rev. 2021, 121, 9554–9643. 10.1021/acs.chemrev.1c00043. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Angala S. K.; Li W.; Boot C. M.; Jackson M.; McNeil M. R. Secondary extended mannan side chains and attachment of the arabinan in mycobacterial lipoarabinomannan. Commun. Chem. 2020, 3, 101. 10.1038/s42004-020-00356-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boltje T. J.; Buskas T.; Boons G.-J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 2009, 1, 611–622. 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adero P. O.; Amarasekara H.; Wen P.; Bohé L.; Crich D. The experimental evidence in support of glycosylation mechanisms at the SN1–SN2 interface. Chem. Rev. 2018, 118, 8242–8284. 10.1021/acs.chemrev.8b00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For selected reviews, see:; a Loh C. C. J. Exploiting non-covalent interactions in selective carbohydrate synthesis. Nat. Rev. Chem. 2021, 5, 792–815. 10.1038/s41570-021-00324-y. [DOI] [PubMed] [Google Scholar]; b Nigudkar S. S.; Demchenko A. V. Stereocontrolled 1,2-cis glycosylation as the driving force of progress in synthetic carbohydrate chemistry. Chem. Sci. 2015, 6, 2687–2704. 10.1039/C5SC00280J. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yao H.; Vu M. D.; Liu X.-W. Recent advances in reagent-controlled stereoselective/stereospecific glycosylation. Carbohydr. Res. 2019, 473, 72–81. 10.1016/j.carres.2018.10.006. [DOI] [PubMed] [Google Scholar]; d Ling J.; Bennett C. S. Recent developments in stereoselective chemical glycosylation. Asian J. Org. Chem. 2019, 8, 802–813. 10.1002/ajoc.201900102. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Warnes M. E.; Fascione M. A. Bimodal glycosyl donors as an emerging approach towards a general glycosylation strategy. Chem.—Eur. J. 2024, 30, e202400399 10.1002/chem.202400399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hettikankanamalage A. A.; Lassfolk R.; Ekholm F. S.; Leino R.; Crich D. Mechanisms of stereodirecting participation and ester migration from near and far in glycosylation and related reactions. Chem. Rev. 2020, 120, 7104–7151. 10.1021/acs.chemrev.0c00243. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Boltje T. J.; Kim J.-H.; Park J.; Boons G.-J. Chiral-auxiliary-mediated 1,2-cis-glycosylations for the solid-supported synthesis of a biologically important branched α-glucan. Nat. Chem. 2010, 2, 552–557. 10.1038/nchem.663. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Le Mai Hoang K.; Liu X.-W. The intriguing dual-directing effect of 2-cyanobenzyl ether for a highly stereospecific glycosylation reaction. Nat. Commun. 2014, 5, 5051. 10.1038/ncomms6051. [DOI] [PubMed] [Google Scholar]; d Elferink H.; Mensink R. A.; White P. B.; Boltje T. J. Stereoselective β-mannosylation by neighboring-group participation. Angew. Chem., Int. Ed. 2016, 55, 11217–11220. 10.1002/anie.201604358. [DOI] [PubMed] [Google Scholar]; e Marianski M.; Mucha E.; Greis K.; Moon S.; Pardo A.; Kirschbaum C.; Thomas D. A.; Meijer G.; von Helden G.; Gilmore K.; Seeberger P. H.; Pagel K. Remote participation during glycosylation reactions of galactose building blocks: direct evidence from cryogenic vibrational spectroscopy. Angew. Chem., Int. Ed. 2020, 59, 6166–6171. 10.1002/anie.201916245. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Upadhyaya K.; Subedi Y. P.; Crich D. Direct experimental characterization of a bridged bicyclic glycosyl dioxacarbenium ion by 1H and 13C NMR spectroscopy: importance of conformation on participation by distal esters. Angew. Chem., Int. Ed. 2021, 60, 25397–25403. 10.1002/anie.202110212. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Liu H.; Hansen T.; Zhou S.-Y.; Wen G.-E.; Liu X.-X.; Zhang Q.-J.; Codée J. D. C.; Schmidt R. R.; Sun J.-S. Dual-participation protecting group solves the anomeric stereocontrol problems in glycosylation reactions. Org. Lett. 2019, 21, 8713–8717. 10.1021/acs.orglett.9b03321. [DOI] [PubMed] [Google Scholar]; h Moons P. H.; ter Braak F.; de Kleijne F. F. J.; Bijleveld B.; Corver S. J. R.; Houthuijs K. J.; Almizori H. R.; Berden G.; Martens J.; Oomens J.; White P. B.; Boltje T. J. Characterization of elusive rhamnosyl dioxanium ions and their application in complex oligosaccharide synthesis. Nat. Commun. 2024, 15, 2257. 10.1038/s41467-024-46522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Duan L.; Nie Q.; Hu Y.; Wang L.; Guo K.; Zhou Z.; Song X.; Tu Y.; Liu H.; Hansen T.; Sun J. S.; Zhang Q. Stereoselective synthesis of the O-antigen of A. baumannii ATCC 17961 using long-range levulinoyl group participation. Angew. Chem., Int. Ed. 2023, 62, e202306971 10.1002/anie.202306971. [DOI] [PubMed] [Google Scholar]; j Liu X.; Song Y.; Liu A.; Zhou Y.; Zhu Q.; Lin Y.; Sun H.; Zhu K.; Liu W.; Ding N.; Xie W.; Sun H.; Yu B.; Xu P.; Li W. More than a leaving group: N-phenyltrifluoroacetimidate as a remote directing group for highly α-selective 1,2-cis glycosylation. Angew. Chem., Int. Ed. 2022, 61, e202201510 10.1002/anie.202201510. [DOI] [PubMed] [Google Scholar]
- a Cumpstey I. Intramolecular aglycon delivery. Carbohydr. Res. 2008, 343, 1553–1573. 10.1016/j.carres.2008.04.031. [DOI] [PubMed] [Google Scholar]; b Mannino M. P.; Demchenko A. V., Hydrogen-bond-mediated aglycone delivery (HAD) and related methods in carbohydrate chemistry. In Carbohydrate Chemistry: Chemical and Biological Approaches; Pilar Rauter A.; Lindhorst T. K.; Queneau Y., Eds.; The Royal Society of Chemistry, 2020; Vol. 44, pp 93–116. [Google Scholar]; c Yasomanee J. P.; Demchenko A. V. Effect of remote picolinyl and picoloyl substituents on the stereoselectivity of chemical glycosylation. J. Am. Chem. Soc. 2012, 134, 20097–20102. 10.1021/ja307355n. [DOI] [PubMed] [Google Scholar]; d Walk J. T.; Buchan Z. A.; Montgomery J. Sugar silanes: versatile reagents for stereocontrolled glycosylation via intramolecular aglycone delivery. Chem. Sci. 2015, 6, 3448–3453. 10.1039/C5SC00810G. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Liu X.; Lin Y.; Peng W.; Zhang Z.; Gao L.; Zhou Y.; Song Z.; Wang Y.; Xu P.; Yu B.; Sun H.; Xie W.; Li W. Direct synthesis of 2,6-dideoxy-β-glycosides and β-rhamnosides with a stereodirecting 2-(diphenylphosphinoyl)acetyl group. Angew. Chem., Int. Ed. 2022, 61, e202206128 10.1002/anie.202206128. [DOI] [PubMed] [Google Scholar]; f Liu Q.-W.; Bin H.-C.; Yang J.-S. β-Arabinofuranosylation using 5-O-(2-quinolinecarbonyl) substituted ethyl thioglycoside donors. Org. Lett. 2013, 15, 3974–3977. 10.1021/ol401755e. [DOI] [PubMed] [Google Scholar]
- a Mulani S. K.; Hung W.-C.; Ingle A. B.; Shiau K.-S.; Mong K.-K. T. Modulating glycosylation with exogenous nucleophiles: an overview. Org. Biomol. Chem. 2014, 12, 1184–1197. 10.1039/c3ob42129e. [DOI] [PubMed] [Google Scholar]; b Lu S. R.; Lai Y. H.; Chen J. H.; Liu C. Y.; Mong K. K. T. Dimethylformamide: an unusual glycosylation modulator. Angew. Chem., Int. Ed. 2011, 50, 7315–7320. 10.1002/anie.201100076. [DOI] [PubMed] [Google Scholar]; c Wang L.; Overkleeft H. S.; van der Marel G. A.; Codée J. D. C. Reagent controlled stereoselective synthesis of α-glucans. J. Am. Chem. Soc. 2018, 140, 4632–4638. 10.1021/jacs.8b00669. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Zhang Y.; He H.; Chen Z.; Huang Y.; Xiang G.; Li P.; Yang X.; Lu G.; Xiao G. Merging reagent modulation and remote anchimeric assistance for glycosylation: highly stereoselective synthesis of α-glycans up to a 30-mer. Angew. Chem., Int. Ed. 2021, 60, 12597–12606. 10.1002/anie.202103826. [DOI] [PubMed] [Google Scholar]; e Yang F.; Hou W.; Zhu D.; Tang Y.; Yu B. A stereoselective glycosylation approach to the construction of 1,2-trans-β-D-glycosidic linkages and convergent synthesis of saponins. Chem.—Eur. J. 2022, 28, e202104002 10.1002/chem.202104002. [DOI] [PubMed] [Google Scholar]; f Yang F.; Sun Y.; Xu P.; Molinaro A.; Silipo A.; Yu B. Synthesis of unprecedented α/β-alternate (1→4)-glucans via stereoselective iterative glycosylation. Chem.—Eur. J. 2023, 29, e202300659 10.1002/chem.202300659. [DOI] [PubMed] [Google Scholar]
- a Guo J.; Ye X.-S. Protecting groups in carbohydrate chemistry: influence on stereoselectivity of glycosylations. Molecules 2010, 15, 7235–7265. 10.3390/molecules15107235. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Crich D. Mechanism of a chemical glycosylation reaction. Acc. Chem. Res. 2010, 43, 1144–1153. 10.1021/ar100035r. [DOI] [PubMed] [Google Scholar]; c Sasaki K.; Uesaki N. Conformationally restricted donors for stereoselective glycosylation. Adv. Carbohydr. Chem. Biochem. 2022, 82, 107–155. 10.1016/bs.accb.2022.10.005. [DOI] [PubMed] [Google Scholar]; d Ikuta D.; Hirata Y.; Wakamori S.; Shimada H.; Tomabechi Y.; Kawasaki Y.; Ikeuchi K.; Hagimori T.; Matsumoto S.; Yamada H. Conformationally supple glucose monomers enable synthesis of the smallest cyclodextrins. Science 2019, 364, 674–677. 10.1126/science.aaw3053. [DOI] [PubMed] [Google Scholar]; e Komura N.; Kato K.; Udagawa T.; Asano S.; Tanaka H.-N.; Imamura A.; Ishida H.; Kiso M.; Ando H. Constrained sialic acid donors enable selective synthesis of α-glycosides. Science 2019, 364, 677–680. 10.1126/science.aaw4866. [DOI] [PubMed] [Google Scholar]
- a Wang H.-Y.; Blaszczyk S. A.; Xiao G.; Tang W. Chiral reagents in glycosylation and modification of carbohydrates. Chem. Soc. Rev. 2018, 47, 681–701. 10.1039/C7CS00432J. [DOI] [PubMed] [Google Scholar]; b Cox D. J.; Smith M. D.; Fairbanks A. J. Glycosylation catalyzed by a chiral brønsted acid. Org. Lett. 2010, 12, 1452–1455. 10.1021/ol1001895. [DOI] [PubMed] [Google Scholar]; c Kimura T.; Sekine M.; Takahashi D.; Toshima K. Chiral brønsted acid mediated glycosylation with recognition of alcohol chirality. Angew. Chem., Int. Ed. 2013, 52, 12131–12134. 10.1002/anie.201304830. [DOI] [PubMed] [Google Scholar]; d Tay J.-H.; Argüelles A. J.; DeMars M. D.; Zimmerman P. M.; Sherman D. H.; Nagorny P. Regiodivergent glycosylations of 6-deoxy-erythronolide B and oleandomycin-derived macrolactones enabled by chiral acid catalysis. J. Am. Chem. Soc. 2017, 139, 8570–8578. 10.1021/jacs.7b03198. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Park Y.; Harper K. C.; Kuhl N.; Kwan E. E.; Liu R. Y.; Jacobsen E. N. Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions. Science 2017, 355, 162–166. 10.1126/science.aal1875. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Levi S. M.; Li Q.; Rötheli A. R.; Jacobsen E. N. Catalytic activation of glycosyl phosphates for stereoselective coupling reactions. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 35–39. 10.1073/pnas.1811186116. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Mayfield A. B.; Metternich J. B.; Trotta A. H.; Jacobsen E. N. Stereospecific furanosylations catalyzed by bis-thiourea hydrogen-bond donors. J. Am. Chem. Soc. 2020, 142, 4061–4069. 10.1021/jacs.0c00335. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Li Q.; Levi S. M.; Jacobsen E. N. Highly selective β-mannosylations and β-rhamnosylations catalyzed by bis-thiourea. J. Am. Chem. Soc. 2020, 142, 11865–11872. 10.1021/jacs.0c04255. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Li Q.; Levi S. M.; Wagen C. C.; Wendlandt A. E.; Jacobsen E. N. Site-selective, stereocontrolled glycosylation of minimally protected sugars. Nature 2022, 608, 74–79. 10.1038/s41586-022-04958-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Peng P.; Schmidt R. R. Acid–base catalysis in glycosidations: a nature derived alternative to the generally employed methodology. Acc. Chem. Res. 2017, 50, 1171–1183. 10.1021/acs.accounts.6b00518. [DOI] [PubMed] [Google Scholar]; b Peng P.; Schmidt R. R. An alternative reaction course in O-glycosidation with O-glycosyl trichloroacetimidates as glycosyl donors and Lewis acidic metal salts as catalyst: acid–base catalysis with gold chloride-glycosyl acceptor adducts. J. Am. Chem. Soc. 2015, 137, 12653–12659. 10.1021/jacs.5b07895. [DOI] [PubMed] [Google Scholar]; c Geng Y.; Kumar A.; Faidallah H. M.; Albar H. A.; Mhkalid I. A.; Schmidt R. R. Cooperative catalysis in glycosidation reactions with O-glycosyl trichloroacetimidates as glycosyl donors. Angew. Chem., Int. Ed. 2013, 52, 10089–10092. 10.1002/anie.201302158. [DOI] [PubMed] [Google Scholar]
- a Takahashi D.; Inaba K.; Toshima K. Recent advances in boron-mediated aglycon delivery (BMAD) for the efficient synthesis of 1,2-cis glycosides. Carbohydr. Res. 2022, 518, 108579. 10.1016/j.carres.2022.108579. [DOI] [PubMed] [Google Scholar]; b Nakagawa A.; Tanaka M.; Hanamura S.; Takahashi D.; Toshima K. Regioselective and 1,2-cis-α-stereoselective glycosylation utilizing glycosyl-acceptor-derived boronic ester catalyst. Angew. Chem., Int. Ed. 2015, 54, 10935–10939. 10.1002/anie.201504182. [DOI] [PubMed] [Google Scholar]; c Tanaka M.; Nakagawa A.; Nishi N.; Iijima K.; Sawa R.; Takahashi D.; Toshima K. Boronic-acid-catalyzed regioselective and 1,2-cis-stereoselective glycosylation of unprotected sugar acceptors via SNi-type mechanism. J. Am. Chem. Soc. 2018, 140, 3644–3651. 10.1021/jacs.7b12108. [DOI] [PubMed] [Google Scholar]; d Inaba K.; Naito Y.; Tachibana M.; Toshima K.; Takahashi D. Regioselective and stereospecific β-arabinofuranosylation by boron-mediated aglycon delivery. Angew. Chem., Int. Ed. 2023, 62, e202307015 10.1002/anie.202307015. [DOI] [PubMed] [Google Scholar]; e Dang Q.-D.; Deng Y.-H.; Sun T.-Y.; Zhang Y.; Li J.; Zhang X.; Wu Y.-D.; Niu D. Catalytic glycosylation for minimally protected donors and acceptors. Nature 2024, 632, 313–319. 10.1038/s41586-024-07695-4. [DOI] [PubMed] [Google Scholar]
- a Li J.; Nguyen H. M. Phenanthroline catalysis in stereoselective 1,2-cis glycosylations. Acc. Chem. Res. 2022, 55, 3738–3751. 10.1021/acs.accounts.2c00636. [DOI] [PubMed] [Google Scholar]; b Xu H.; Schaugaard R. N.; Li J.; Schlegel H. B.; Nguyen H. M. Stereoselective 1,2-cis furanosylations catalyzed by phenanthroline. J. Am. Chem. Soc. 2022, 144, 7441–7456. 10.1021/jacs.2c02063. [DOI] [PMC free article] [PubMed] [Google Scholar]; c DeMent P. M.; Liu C.; Wakpal J.; Schaugaard R. N.; Schlegel H. B.; Nguyen H. M. Phenanthroline-catalyzed stereoselective formation of α-1,2-cis 2-deoxy-2-fluoro glycosides. ACS Catal. 2021, 11, 2108–2120. 10.1021/acscatal.0c04381. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Yu F.; Li J.; DeMent P. M.; Tu Y. J.; Schlegel H. B.; Nguyen H. M. Phenanthroline-catalyzed stereoretentive glycosylations. Angew. Chem., Int. Ed. 2019, 58, 6957–6961. 10.1002/anie.201901346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Deng L.-F.; Wang Y.; Xu S.; Shen A.; Zhu H.; Zhang S.; Zhang X.; Niu D. Palladium catalysis enables cross-coupling–like SN2-glycosylation of phenols. Science 2023, 382, 928–935. 10.1126/science.adk1111. [DOI] [PubMed] [Google Scholar]
- For selected miscellaneous reports, see:; a Nguyen H.; Zhu D.; Li X.; Zhu J. Stereoselective construction of β-mannopyranosides by anomeric O-alkylation: synthesis of the trisaccharide core of N-linked glycans. Angew. Chem., Int. Ed. 2016, 55, 4767–4771. 10.1002/anie.201600488. [DOI] [PubMed] [Google Scholar]; b Hoang K. M.; Lees N. R.; Herzon S. B. General method for the synthesis of α- or β-deoxyaminoglycosides bearing basic nitrogen. J. Am. Chem. Soc. 2021, 143, 2777–2783. 10.1021/jacs.0c11262. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Wu J.; Jia P.; Kuniyil R.; Liu P.; Tang W. Dynamic kinetic stereoselective glycosylation via RhII and chiral phosphoric acid-cocatalyzed carbenoid insertion to the anomeric OH bond for the synthesis of glycoconjugates. Angew. Chem., Int. Ed. 2023, 62, e202307144 10.1002/anie.202307144. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li T.-R.; Piccini G.; Tiefenbacher K. Supramolecular capsule-catalyzed highly β-selective furanosylation independent of the SN1/SN2 reaction pathway. J. Am. Chem. Soc. 2023, 145, 4294–4303. 10.1021/jacs.2c13641. [DOI] [PubMed] [Google Scholar]; e Balmond E. I.; Coe D. M.; Galan M. C.; McGarrigle E. M. α-Selective organocatalytic synthesis of 2-deoxygalactosides. Angew. Chem., Int. Ed. 2012, 51, 9152–9155. 10.1002/anie.201204505. [DOI] [PubMed] [Google Scholar]; f Sun L.; Wu X.; Xiong D. C.; Ye X. S. Stereoselective Koenigs–Knorr glycosylation catalyzed by urea. Angew. Chem., Int. Ed. 2016, 55, 8041–8044. 10.1002/anie.201600142. [DOI] [PubMed] [Google Scholar]
- a Nielsen M. M.; Pedersen C. M. Catalytic glycosylations in oligosaccharide synthesis. Chem. Rev. 2018, 118, 8285–8358. 10.1021/acs.chemrev.8b00144. [DOI] [PubMed] [Google Scholar]; b Crawford C. J.; Seeberger P. H. Advances in glycoside and oligosaccharide synthesis. Chem. Soc. Rev. 2023, 52, 7773–7801. 10.1039/D3CS00321C. [DOI] [PubMed] [Google Scholar]
- Campeau D.; León Rayo D. F.; Mansour A.; Muratov K.; Gagosz F. Gold-catalyzed reactions of specially activated alkynes, allenes, and alkenes. Chem. Rev. 2021, 121, 8756–8867. 10.1021/acs.chemrev.0c00788. [DOI] [PubMed] [Google Scholar]
- a Li Y.; Yang Y.; Yu B. An efficient glycosylation protocol with glycosyl ortho-alkynylbenzoates as donors under the catalysis of Ph3PAuOTf. Tetrahedron Lett. 2008, 49, 3604–3608. 10.1016/j.tetlet.2008.04.017. [DOI] [Google Scholar]; b Yu B. Gold(I)-catalyzed glycosylation with glycosyl o-alkynylbenzoates as donors. Acc. Chem. Res. 2018, 51, 507–516. 10.1021/acs.accounts.7b00573. [DOI] [PubMed] [Google Scholar]
- For recent reports on the synthesis of complex carbohydrates, see:; a Zhang Z.; Wu R.; Cao S.; Li J.; Huang G.; Wang H.; Yang T.; Tang W.; Xu P.; Yu B. Merging total synthesis and NMR technology for deciphering the realistic structure of natural 2,6-dideoxyglycosides. Sci. Adv. 2024, 10, eadn1305 10.1126/sciadv.adn1305. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Chen Z.; Xiao G. Total synthesis of nona-decasaccharide motif from Ganoderma sinense polysaccharide enabled by modular and one-pot stereoselective glycosylation strategy. J. Am. Chem. Soc. 2024, 146, 17446–17455. 10.1021/jacs.4c05188. [DOI] [PubMed] [Google Scholar]; c Ma Y.; Zhang Y.; Huang Y.; Chen Z.; Xian Q.; Su R.; Jiang Q.; Wang X.; Xiao G. One-pot assembly of mannose-capped lipoarabinomannan motifs up to 101-mer from the Mycobacterium tuberculosis cell wall. J. Am. Chem. Soc. 2024, 146, 4112–4122. 10.1021/jacs.3c12815. [DOI] [PubMed] [Google Scholar]; d Zhang Y.; Wang L.; Zhou Q.; Li Z.; Li D.; Yin C.; Wang X.; Xiao G. Modular synthesis of a tridecasaccharide motif of Bacteroides vulgatus lipopolysaccharides against inflammatory bowel diseases through an orthogonal one-pot glycosylation strategy. Angew. Chem., Int. Ed. 2023, 62, e202301351 10.1002/anie.202301351. [DOI] [PubMed] [Google Scholar]; e Wang J.; Gao J.; Guo T.; Huo X.; Zhang W.; Liu J.; Wang X. Bioinspired total synthesis of complex nucleoside antibiotics A201A, A201D and A201E. Angew. Chem., Int. Ed. 2023, 62, e202213810 10.1002/anie.202213810. [DOI] [PubMed] [Google Scholar]; f Zhu D.; Geng M.; Yu B. Total synthesis of starfish cyclic steroid glycosides. Angew. Chem., Int. Ed. 2022, 61, e202203239 10.1002/anie.202203239. [DOI] [PubMed] [Google Scholar]; g Shao X.; Zheng C.; Xu P.; Shiraishi T.; Kuzuyama T.; Molinaro A.; Silipo A.; Yu B. Total synthesis and stereochemistry assignment of nucleoside antibiotic A-94964. Angew. Chem., Int. Ed. 2022, 61, e202200818 10.1002/anie.202200818. [DOI] [PubMed] [Google Scholar]
- a Zhu Y.; Yu B. Highly stereoselective β-mannopyranosylation via the 1-α-glycosyloxy-isochromenylium-4-gold(I) intermediates. Chem.—Eur. J. 2015, 21, 8771–8780. 10.1002/chem.201500648. [DOI] [PubMed] [Google Scholar]; b Zhu Y.; Shen Z.; Li W.; Yu B. Stereoselective synthesis of β-rhamnopyranosides via gold(I)-catalyzed glycosylation with 2-alkynyl-4-nitro-benzoate donors. Org. Biomol. Chem. 2016, 14, 1536–1539. 10.1039/C5OB02551F. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Li J.; Zhu Y.; Li Y.; Yu B. Mechanistic insights into the gold(I)-catalyzed activation of glycosyl ortho-alkynylbenzoates for glycosidation. J. Am. Chem. Soc. 2013, 135, 18396–18405. 10.1021/ja4064316. [DOI] [PubMed] [Google Scholar]
- Zeng J.; Wang R.; Zhang S.; Fang J.; Liu S.; Sun G.; Xu B.; Xiao Y.; Fu D.; Zhang W.; Hu Y.; Wan Q. Hydrogen-bonding-assisted exogenous nucleophilic reagent effect for β-selective glycosylation of rare 3-amino sugars. J. Am. Chem. Soc. 2019, 141, 8509–8515. 10.1021/jacs.9b01862. [DOI] [PubMed] [Google Scholar]
- a Zhang Y.; Ma X.; Zhang L. Highly stereoselective synthesis of 2-azido-2-deoxyglycosides via gold-catalyzed SN2 glycosylation. CCS Chem. 2023, 5, 2799–2807. 10.31635/ccschem.023.202303086. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ma X.; Zhang Y.; Zhu X.; Wei Y.; Zhang L. Directed SN2 glycosylation employing an amide-functionalized 1-naphthoate platform featuring a selectivity-safeguarding mechanism. J. Am. Chem. Soc. 2023, 145, 11921–11926. 10.1021/jacs.3c02792. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ma X.; Zhang Y.; Zhu X.; Zhang L. An SN2-type strategy toward 1,2-cis-furanosides. CCS Chem. 2022, 4, 3677–3685. 10.31635/ccschem.022.202202175. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Ma X.; Zheng Z.; Fu Y.; Zhu X.; Liu P.; Zhang L. A “traceless” directing group enables catalytic SN2 glycosylation toward 1,2-cis-glycopyranosides. J. Am. Chem. Soc. 2021, 143, 11908–11913. 10.1021/jacs.1c04584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.; Zhou Y.; Wang Y.; Lin Y.; Pan S.; Che Q.; Sang J.; Gao Z.; Zhang W.; Wang Y.; Li G.; Gao L.; Wang Z.; Yang X.; Liu A.; Wang S.; Yu B.; Xu P.; Wang Z.; Zhang Z.; Yang P.; Xie W.; Sun H.; Li W. Direct synthesis of α- and β-2′-deoxynucleosides with stereodirecting phosphine oxide via remote participation. J. Am. Chem. Soc. 2024, 146, 8768–8779. 10.1021/jacs.4c01780. [DOI] [PubMed] [Google Scholar]
- a Martí À.; Ogalla G.; Echavarren A. M. Hydrogen-bonded matched ion pair gold(I) catalysis. ACS Catal. 2023, 13, 10217–10223. 10.1021/acscatal.3c02638. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Franchino A.; Martí À.; Echavarren A. M. H-bonded counterion-directed enantioselective Au(I) catalysis. J. Am. Chem. Soc. 2022, 144, 3497–3509. 10.1021/jacs.1c11978. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Franchino A.; Martí À.; Nejrotti S.; Echavarren A. M. Silver-free Au(I) catalysis enabled by bifunctional urea- and squaramide-phosphine ligands via H-bonding. Chem.—Eur. J. 2021, 27, 11989–11996. 10.1002/chem.202101751. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Campbell M. J.; Toste F. D. Enantioselective synthesis of cyclic carbamimidates via a three-component reaction of imines, terminal alkynes, and p-toluenesulfonylisocyanate using a monophosphine gold(I) catalyst. Chem. Sci. 2011, 2, 1369–1378. 10.1039/c1sc00160d. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Martí À.; Montesinos-Magraner M.; Echavarren A. M.; Franchino A. H-bonded counterion-directed catalysis: enantioselective gold(I)-catalyzed addition to 2-alkynyl enones as a case study. Eur. J. Org. Chem. 2022, 2022, e202200518 10.1002/ejoc.202200518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z.; Li T.; Mudshinge S. R.; Xu B.; Hammond G. B. Optimization of catalysts and conditions in gold(I) catalysis—counterion and additive effects. Chem. Rev. 2021, 121, 8452–8477. 10.1021/acs.chemrev.0c00713. [DOI] [PubMed] [Google Scholar]
- a Qureshi N.; Yufit D. S.; Steed K. M.; Howard J. A. K.; Steed J. W. Anion hydrogen bonding from a ‘revealed’ urea ligand. CrystEngComm 2016, 18, 5333–5337. 10.1039/C6CE01039C. [DOI] [Google Scholar]; b Keszei S. J.; Balogh S.; Fehér C.; Nagy L.; Tumanov N.; Wouters J.; Lendvay G.; Skoda-Földes R. Molecular recognition of strong acids by using a 2-ureido-4-ferrocenyl pyrimidine receptor. Eur. J. Inorg. Chem. 2019, 2019, 4095–4104. 10.1002/ejic.201900803. [DOI] [Google Scholar]; c Homs A.; Escofet I.; Echavarren A. M. On the silver effect and the formation of chloride-bridged digold complexes. Org. Lett. 2013, 15, 5782–5785. 10.1021/ol402825v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ovian J. M.; Vojáčková P.; Jacobsen E. N. Enantioselective transition-metal catalysis via an anion-binding approach. Nature 2023, 616, 84–89. 10.1038/s41586-023-05804-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Banik S. M.; Levina A.; Hyde A. M.; Jacobsen E. N. Lewis acid enhancement by hydrogen-bond donors for asymmetric catalysis. Science 2017, 358, 761–764. 10.1126/science.aao5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Zuo H.; Zhang C.; Zhang Y.; Niu D. Base-promoted glycosylation allows protecting group-free and stereoselective O-glycosylation of carboxylic acids. Angew. Chem., Int. Ed. 2023, 62, e202309887 10.1002/anie.202309887. [DOI] [PubMed] [Google Scholar]; b Liu Z.; Liu D.; Zhu D.; Yu B. Stereoselective synthesis of β-glycosyl esters via 1-hydroxybenzotriazole mediated acylation of glycosyl hemiacetals. Org. Lett. 2023, 25, 5372–5377. 10.1021/acs.orglett.3c01750. [DOI] [PubMed] [Google Scholar]; c Wang H.-Y.; Simmons C. J.; Zhang Y.; Smits A. M.; Balzer P. G.; Wang S.; Tang W. Chiral catalyst-directed dynamic kinetic diastereoselective acylation of anomeric hydroxyl groups and a controlled reduction of the glycosyl ester products. Org. Lett. 2017, 19, 508–511. 10.1021/acs.orglett.6b03683. [DOI] [PubMed] [Google Scholar]; d Zhao C.; Li F.; Wang J. N-heterocyclic carbene catalyzed dynamic kinetic resolution of pyranones. Angew. Chem., Int. Ed. 2016, 55, 1820–1824. 10.1002/anie.201508205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.