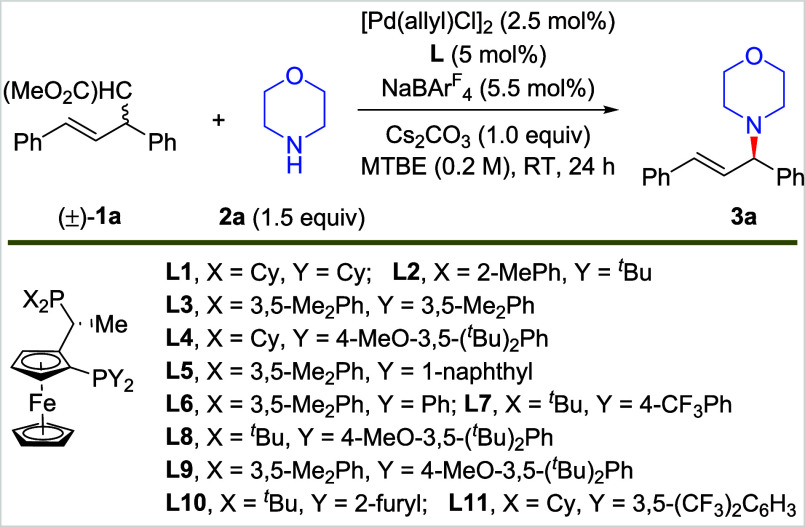
Table 1. Reaction Development for Asymmetric C–C Amination.
| Entrya | L | Solvent | Yield (%) | er |
|---|---|---|---|---|
| 1 | L1 | MTBE | 37 | 50:50 |
| 2 | L2 | MTBE | 4 | 55:45 |
| 3 | L3 | MTBE | 10 | 30:70 |
| 4 | L4 | MTBE | 32 | 75:25 |
| 5 | L5 | MTBE | 34 | 19:81 |
| 6 | L6 | MTBE | 30 | 72:28 |
| 7 | L7 | MTBE | 41 | 69:31 |
| 8 | L8 | MTBE | 76 | 40:60 |
| 9 | L9 | MTBE | 12 | 75:25 |
| 10 | L10 | MTBE | 72 | 58:42 |
| 11 | L11 | MTBE | 10 | 94:6 |
| 12 | L11 | MTBE | 22 | 95:5 |
| 13 | L11 | cyclohexane | 61 | 90:10 |
| 14 | L11 | mesitylene | 69 | 82:18 |
| 15 | L11 | MeOH | 18 | 53:47 |
| 16b, | L11 | PhEt | 56 | 91:9 |
| 17b,c, | L11 | PhEt | 86 | 95:5 |
The reaction was carried out in 0.10 mmol scale. The yield was determined by 1H NMR. The er was determined by HPLC analysis.
2a (3 equiv) was used.
PhEt (0.5 M) was used as the solvent.
KOtBu was used instead of Cs2CO3. Isolated yield.